Combination of the Immune Modulator Fingolimod With Alteplase in Acute Ischemic Stroke: A Pilot Trial
- PMID: 26202811
- PMCID: PMC4580515
- DOI: 10.1161/CIRCULATIONAHA.115.016371
Combination of the Immune Modulator Fingolimod With Alteplase in Acute Ischemic Stroke: A Pilot Trial
Abstract
Background: Inflammatory and immune responses triggered by brain ischemia worsen clinical outcomes of stroke and contribute to hemorrhagic transformation, massive edema, and reperfusion injury associated with intravenous alteplase. We assessed whether a combination of the immune-modulator fingolimod and alteplase is safe and effective in attenuating reperfusion injury in patients with acute ischemic stroke treated within the first 4.5 hours of symptom onset.
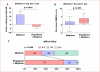
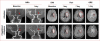
Methods and results: In this multicenter trial, we randomly assigned 25 eligible patients with hemispheric ischemic stroke stemming from anterior or middle cerebral arterial occlusion to receive alteplase alone and 22 patients to receive alteplase plus oral fingolimod 0.5 mg daily for 3 consecutive days within 4.5 hours of the onset of ischemic stroke. Compared with patients who received alteplase alone, patients who received the combination of fingolimod with alteplase exhibited lower circulating lymphocytes, smaller lesion volumes (10.1 versus 34.3 mL; P=0.04), less hemorrhage (1.2 versus 4.4 mL; P=0.01), and attenuated neurological deficits in National Institute of Health Stroke Scales (4 versus 2; P=0.02) at day 1. Furthermore, restrained lesion growth from day 1 to 7 (-2.3 versus 12.1 mL; P<0.01) with a better recovery at day 90 (modified Rankin Scale score 0-1, 73% versus 32%; P<0.01) was evident in patients given fingolimod and alteplase. No serious adverse events were recorded in all patients.
Conclusions: In this pilot study, combination therapy of fingolimod and alteplase was well tolerated, attenuated reperfusion injury, and improved clinical outcomes in patients with acute ischemic stroke. These findings need to be tested in further clinical trials.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT02002390.
Keywords: brain ischemia; edema; hemorrhage; inflammation; thrombolytic therapy.
© 2015 American Heart Association, Inc.
Figures



References
-
- Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D, ECASS Investigators Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. - PubMed
-
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. - PubMed
-
- The NINDS t-PA Stroke Study Group Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28:2109–2118. - PubMed
-
- Jaillard A, Cornu C, Durieux A, Moulin T, Boutitie F, Lees KR, Hommel M. Hemorrhagic transformation in acute ischemic stroke. The MAST-E study. MAST-E Group. Stroke. 1999;30:1326–1332. - PubMed
-
- Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, Lee KH, Liebeskind DS, UCLA-Samsung Stroke Collaborators Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke. 2011;42:2235–2239. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

