Fe(2+) substrate transport through ferritin protein cage ion channels influences enzyme activity and biomineralization
- PMID: 26202907
- PMCID: PMC4634868
- DOI: 10.1007/s00775-015-1279-x
Fe(2+) substrate transport through ferritin protein cage ion channels influences enzyme activity and biomineralization
Abstract
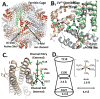
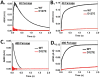
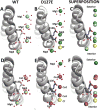
Ferritins, complex protein nanocages, form internal iron-oxy minerals (Fe2O3·H2O), by moving cytoplasmic Fe(2+) through intracage ion channels to cage-embedded enzyme (2Fe(2+)/O2 oxidoreductase) sites where ferritin biomineralization is initiated. The products of ferritin enzyme activity are diferric oxy complexes that are mineral precursors. Conserved, carboxylate amino acid side chains of D127 from each of three cage subunits project into ferritin ion channels near the interior ion channel exits and, thus, could direct Fe(2+) movement to the internal enzyme sites. Ferritin D127E was designed and analyzed to probe properties of ion channel size and carboxylate crowding near the internal ion channel opening. Glu side chains are chemically equivalent to, but longer by one -CH2 than Asp, side chains. Ferritin D127E assembled into normal protein cages, but diferric peroxo formation (enzyme activity) was not observed, when measured at 650 nm (DFP λ max). The caged biomineral formation, measured at 350 nm in the middle of the broad, nonspecific Fe(3+)-O absorption band, was slower. Structural differences (protein X-ray crystallography), between ion channels in wild type and ferritin D127E, which correlate with the inhibition of ferritin D127E enzyme activity include: (1) narrower interior ion channel openings/pores; (2) increased numbers of ion channel protein-metal binding sites, and (3) a change in ion channel electrostatics due to carboxylate crowding. The contributions of ion channel size and structure to ferritin activity reflect metal ion transport in ion channels are precisely regulated both in ferritin protein nanocages and membranes of living cells.
Figures

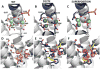
Similar articles
-
Solving Biology's Iron Chemistry Problem with Ferritin Protein Nanocages.Acc Chem Res. 2016 May 17;49(5):784-91. doi: 10.1021/ar500469e. Epub 2016 May 2. Acc Chem Res. 2016. PMID: 27136423
-
Moving Fe2+ from ferritin ion channels to catalytic OH centers depends on conserved protein cage carboxylates.Proc Natl Acad Sci U S A. 2014 Jun 3;111(22):7925-30. doi: 10.1073/pnas.1318417111. Epub 2014 May 19. Proc Natl Acad Sci U S A. 2014. PMID: 24843174 Free PMC article.
-
Ferritin ion channel disorder inhibits Fe(II)/O2 reactivity at distant sites.Inorg Chem. 2012 Nov 5;51(21):11406-11. doi: 10.1021/ic3010135. Epub 2012 Oct 23. Inorg Chem. 2012. PMID: 23092300 Free PMC article.
-
Ferritin protein nanocages use ion channels, catalytic sites, and nucleation channels to manage iron/oxygen chemistry.Curr Opin Chem Biol. 2011 Apr;15(2):304-11. doi: 10.1016/j.cbpa.2011.01.004. Epub 2011 Feb 4. Curr Opin Chem Biol. 2011. PMID: 21296609 Free PMC article. Review.
-
Ferritin: the protein nanocage and iron biomineral in health and in disease.Inorg Chem. 2013 Nov 4;52(21):12223-33. doi: 10.1021/ic400484n. Epub 2013 Oct 8. Inorg Chem. 2013. PMID: 24102308 Free PMC article. Review.
Cited by
-
Design of a gold clustering site in an engineered apo-ferritin cage.Commun Chem. 2022 Mar 21;5(1):39. doi: 10.1038/s42004-022-00651-1. Commun Chem. 2022. PMID: 36697940 Free PMC article.
-
How can sodium-glucose cotransporter 2 inhibitors stimulate erythrocytosis in patients who are iron-deficient? Implications for understanding iron homeostasis in heart failure.Eur J Heart Fail. 2022 Dec;24(12):2287-2296. doi: 10.1002/ejhf.2731. Epub 2022 Nov 21. Eur J Heart Fail. 2022. PMID: 36377108 Free PMC article. Review.
-
Genome-wide identification and expression analysis of the ferritin family in sweetpotato and its two diploid relatives.BMC Plant Biol. 2025 Jun 5;25(1):765. doi: 10.1186/s12870-025-06732-2. BMC Plant Biol. 2025. PMID: 40474077 Free PMC article.
-
Perls' Prussian blue staining and chemistry of Prussian blue and Turnbull blue.Forensic Sci Int Synerg. 2025 Jul 10;11:100627. doi: 10.1016/j.fsisyn.2025.100627. eCollection 2025 Dec. Forensic Sci Int Synerg. 2025. PMID: 40686580 Free PMC article. Review.
-
Structural and Functional Insights into the Roles of Potential Metal-Binding Sites in Apostichopus japonicus Ferritin.Polymers (Basel). 2022 Dec 8;14(24):5378. doi: 10.3390/polym14245378. Polymers (Basel). 2022. PMID: 36559745 Free PMC article.
References
-
- Treffry A, Zhao Z, Quail MA, Guest JR, Harrison PM. Biochemistry. 1995;34:15204–15213. - PubMed
-
- Zhao Z, Treffry A, A Quail M, R Guest J, M Harrison P. Journal of the Chemical Society, Dalton Transactions. 1997:3977–3978.
-
- Chasteen ND, Harrison PM. J Struct Biol. 1999;126:182–194. - PubMed
-
- de Val N, Declercq JP, Lim CK, Crichton RR. Journal of inorganic biochemistry. 2012;112:77–84. - PubMed
-
- Granier T, Langlois d'Estaintot B, Gallois B, Chevalier JM, Precigoux G, Santambrogio P, Arosio P. J Biol Inorg Chem. 2003;8:105–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

