Transforming growth factor-β1 up-regulates connexin43 expression in human granulosa cells
- PMID: 26202915
- PMCID: PMC4542719
- DOI: 10.1093/humrep/dev175
Transforming growth factor-β1 up-regulates connexin43 expression in human granulosa cells
Abstract
Study question: Does transforming growth factor-β1 (TGF-β1) up-regulate connexin43 (Cx43) to promote cell-cell communication in human granulosa cells?
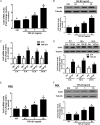
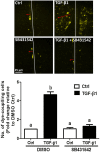
Summary answer: TGF-β1 up-regulates Cx43 and increases gap junction intercellular communication activities (GJIC) in human granulosa cells, and this effect occurs via the activin receptor-like kinase (ALK)5-mediated Sma- and Mad-related protein (SMAD)2/3-SMAD4-dependent pathway.
What is known already: TGF-β1 and its receptors are expressed in human granulosa cells, and follicular fluid contains TGF-β1 protein. In human granulosa cells, Cx43 gap junctions play an important role in the development of follicles and oocytes.
Study design, size, duration: This is an experimental study which was performed over a 1-year period.
Participants/materials, setting, methods: Immortalized human granulosa cells (SVOG cells) and primary human granulosa-lutein cells obtained from women undergoing IVF in an academic research center were used as the study models. Cx43 mRNA and protein expression levels were examined after exposure of SVOG cells to recombinant human TGF-β1. An activin/TGF-β type I receptor inhibitor, SB431542, and small interfering RNAs targeting ALK4, ALK5, SMAD2, SMAD3 and SMAD4 were used to verify the specificity of the effects and to investigate the molecular mechanisms. Real-time-quantitative PCR and western blot analysis were used to detect the specific mRNA and protein levels, respectively. GJIC between SVOG cells were evaluated using a scrape loading and dye transfer assay. Results were analyzed by one-way analysis of variance.
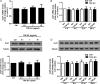
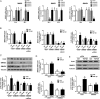
Main results and the role of chance: TGF-β1 treatment increased phosphorylation of SMAD2/3 (P < 0.0001) and up-regulated Cx43 mRNA and protein levels (P < 0.001) in SVOG cells and these stimulatory effects were abolished by the TGF-β type I receptor inhibitor SB431542. In addition, the up-regulatory effect of TGF-β1 on Cx43 expression (mRNA and protein) was confirmed in primary cultures of human granulosa-lutein cells (P < 0.05). The small interfering RNA-mediated knockdown of ALK5, but not ALK4, abolished the TGF-β1-induced phosphorylation of SMAD2/3 and the up-regulation of Cx43. Furthermore, knockdown of SMAD2/3 or the common SMAD, SMAD4, abolished the stimulatory effects of TGF-β1 on Cx43 expression in SVOG cells. The TGF-β1-induced up-regulation of Cx43 contributed to the increase of GJIC between SVOG cells (P < 0.001).
Limitations, reasons for caution: The results of this study were generated from in vitro system and may not reflect the intra-ovarian microenvironment in vivo.
Wider implications of the findings: Our studies represent the first comprehensive research of molecular mechanisms of TGF-β1 in the regulation of Cx43 expression and GJIC in human granulosa cells and demonstrate that TGF-β1 may play a crucial role in the local modulation of cell-cell communication. Deepening our understanding of the molecular determinants will offer important insights into ovarian physiology and lead to the development of potential therapeutic methods for fertility regulation.
Study funding/competing interests: This research was supported by an operating grant from the Canadian Institutes of Health Research to P.C.K.L. There are no conflicts of interest to declare.
Trial registration number: NA.
Keywords: ALK5; SMAD; connexin43; human granulosa cells; transforming growth factor-β1.
© The Author 2015. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


Similar articles
-
Bone morphogenetic protein 2 regulates cell-cell communication by down-regulating connexin43 expression in luteinized human granulosa cells.Mol Hum Reprod. 2017 Mar 1;23(3):155-165. doi: 10.1093/molehr/gaw078. Mol Hum Reprod. 2017. PMID: 27986931
-
Theca-derived BMP4 and BMP7 down-regulate connexin43 expression and decrease gap junction intercellular communication activity in immortalized human granulosa cells.J Clin Endocrinol Metab. 2013 Mar;98(3):E437-45. doi: 10.1210/jc.2012-3851. Epub 2013 Feb 5. J Clin Endocrinol Metab. 2013. PMID: 23386650
-
Oocyte-derived BMP15 but not GDF9 down-regulates connexin43 expression and decreases gap junction intercellular communication activity in immortalized human granulosa cells.Mol Hum Reprod. 2014 May;20(5):373-83. doi: 10.1093/molehr/gau001. Epub 2014 Jan 10. Mol Hum Reprod. 2014. PMID: 24413384 Free PMC article.
-
The transforming growth factor beta 1/SMAD signaling pathway involved in human chronic myeloid leukemia.Tumori. 2010 Sep-Oct;96(5):659-66. doi: 10.1177/030089161009600503. Tumori. 2010. PMID: 21302608 Review.
-
Cell-to-cell communication in the ovarian follicle: developmental and hormonal regulation of the expression of connexin43.Hum Reprod. 1998 Dec;13 Suppl 4:85-97. doi: 10.1093/humrep/13.suppl_4.85. Hum Reprod. 1998. PMID: 10091060 Review.
Cited by
-
Transforming growth factor-β1 up-regulates connexin43 expression in osteocytes via canonical Smad-dependent signaling pathway.Biosci Rep. 2018 Dec 14;38(6):BSR20181678. doi: 10.1042/BSR20181678. Print 2018 Dec 21. Biosci Rep. 2018. PMID: 30482881 Free PMC article.
-
The genomic response of human granulosa cells (KGN) to melatonin and specific agonists/antagonists to the melatonin receptors.Sci Rep. 2022 Oct 20;12(1):17539. doi: 10.1038/s41598-022-21162-y. Sci Rep. 2022. PMID: 36266374 Free PMC article.
-
Transforming growth factor-β1-induced N-cadherin drives cell-cell communication through connexin43 in osteoblast lineage.Int J Oral Sci. 2021 Apr 13;13(1):15. doi: 10.1038/s41368-021-00119-3. Int J Oral Sci. 2021. PMID: 33850101 Free PMC article.
-
Cx43 upregulation in HUVECs under stretch via TGF-β1 and cytoskeletal network.Open Med (Wars). 2022 Mar 9;17(1):463-474. doi: 10.1515/med-2022-0432. eCollection 2022. Open Med (Wars). 2022. PMID: 35350835 Free PMC article.
-
Transforming Growth Factor-beta 1 Involved in the Pathogenesis of Endometriosis through Regulating Expression of Vascular Endothelial Growth Factor under Hypoxia.Chin Med J (Engl). 2017 Apr 20;130(8):950-956. doi: 10.4103/0366-6999.204112. Chin Med J (Engl). 2017. PMID: 28397725 Free PMC article.
References
-
- Ackert CL, Gittens JE, O'Brien MJ, Eppig JJ, Kidder GM. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol 2001;233:258–270. - PubMed
-
- Beyer EC, Paul DL, Goodenough DA. Connexin family of gap junction proteins. J Membr Biol 1990;116:187–194. - PubMed
-
- Brown KA, Pietenpol JA, Moses HL. A tale of two proteins: differential roles and regulation of Smad2 and Smad3 in TGF-beta signaling. J Cell Biochem 2007;101:9–33. - PubMed
-
- Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem 1996;238:1–27. - PubMed
-
- Casari A, Schiavone M, Facchinello N, Vettori A, Meyer D, Tiso N, Moro E, Argenton F. A Smad3 transgenic reporter reveals TGF-beta control of zebrafish spinal cord development. Dev Biol 2014;396:81–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

