β-Catenin is required for intrinsic but not extrinsic BCR-ABL1 kinase-independent resistance to tyrosine kinase inhibitors in chronic myeloid leukemia
- PMID: 26202934
- PMCID: PMC4675686
- DOI: 10.1038/leu.2015.196
β-Catenin is required for intrinsic but not extrinsic BCR-ABL1 kinase-independent resistance to tyrosine kinase inhibitors in chronic myeloid leukemia
Abstract
Activation of nuclear β-catenin and expression of its transcriptional targets promotes chronic myeloid leukemia (CML) progression, tyrosine kinase inhibitor (TKI) resistance, and leukemic stem cell self-renewal. We report that nuclear β-catenin has a role in leukemia cell-intrinsic but not -extrinsic BCR-ABL1 kinase-independent TKI resistance. Upon imatinib inhibition of BCR-ABL1 kinase activity, β-catenin expression was maintained in intrinsically resistant cells grown in suspension culture and sensitive cells cultured in direct contact (DC) with bone marrow (BM) stromal cells. Thus, TKI resistance uncouples β-catenin expression from BCR-ABL1 kinase activity. In β-catenin reporter assays, intrinsically resistant cells showed increased transcriptional activity versus parental TKI-sensitive controls, and this was associated with restored expression of β-catenin target genes. In contrast, DC with BM stromal cells promoted TKI resistance, but had little effects on Lef/Tcf reporter activity and no consistent effects on cytoplasmic β-catenin levels, arguing against a role for β-catenin in extrinsic TKI resistance. N-cadherin or H-cadherin blocking antibodies abrogated DC-based resistance despite increasing Lef/Tcf reporter activity, suggesting that factors other than β-catenin contribute to extrinsic, BM-derived TKI resistance. Our data indicate that, while nuclear β-catenin enhances survival of intrinsically TKI-resistant CML progenitors, it is not required for extrinsic resistance mediated by the BM microenvironment.
Conflict of interest statement
Figures

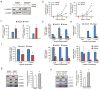
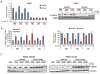


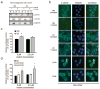
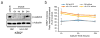
Similar articles
-
PP2A-activating drugs selectively eradicate TKI-resistant chronic myeloid leukemic stem cells.J Clin Invest. 2013 Oct;123(10):4144-57. doi: 10.1172/JCI68951. Epub 2013 Sep 3. J Clin Invest. 2013. PMID: 23999433 Free PMC article.
-
Microenvironmental protection of CML stem and progenitor cells from tyrosine kinase inhibitors through N-cadherin and Wnt-β-catenin signaling.Blood. 2013 Mar 7;121(10):1824-38. doi: 10.1182/blood-2012-02-412890. Epub 2013 Jan 8. Blood. 2013. PMID: 23299311 Free PMC article.
-
Combined STAT3 and BCR-ABL1 inhibition induces synthetic lethality in therapy-resistant chronic myeloid leukemia.Leukemia. 2015 Mar;29(3):586-597. doi: 10.1038/leu.2014.245. Epub 2014 Aug 19. Leukemia. 2015. PMID: 25134459 Free PMC article.
-
[MET/ERK and MET/JNK Pathway Activation Is Involved in BCR-ABL Inhibitor-resistance in Chronic Myeloid Leukemia].Yakugaku Zasshi. 2018;138(12):1461-1466. doi: 10.1248/yakushi.18-00142. Yakugaku Zasshi. 2018. PMID: 30504658 Review. Japanese.
-
Reactive oxygen species in BCR-ABL1-expressing cells - relevance to chronic myeloid leukemia.Acta Biochim Pol. 2017;64(1):1-10. doi: 10.18388/abp.2016_1396. Epub 2016 Dec 1. Acta Biochim Pol. 2017. PMID: 27904889 Review.
Cited by
-
Involvement of Oxidative Stress in Resistance to Tyrosine-Kinase Inhibitors Therapy in Chronic Myeloid Leukemia.Curr Health Sci J. 2020 Oct-Dec;46(4):420-432. doi: 10.12865/CHSJ.46.04.14. Epub 2020 Dec 31. Curr Health Sci J. 2020. PMID: 33717518 Free PMC article.
-
An Overview of Myeloid Blast-Phase Chronic Myeloid Leukemia.Cancers (Basel). 2024 Oct 26;16(21):3615. doi: 10.3390/cancers16213615. Cancers (Basel). 2024. PMID: 39518058 Free PMC article. Review.
-
Proteasome 26S subunit, non-ATPases 1 (PSMD1) and 3 (PSMD3), play an oncogenic role in chronic myeloid leukemia by stabilizing nuclear factor-kappa B.Oncogene. 2021 Apr;40(15):2697-2710. doi: 10.1038/s41388-021-01732-6. Epub 2021 Mar 12. Oncogene. 2021. PMID: 33712704 Free PMC article.
-
Low c-Kit expression identifies primitive, therapy-resistant CML stem cells.JCI Insight. 2023 Jan 10;8(1):e157421. doi: 10.1172/jci.insight.157421. JCI Insight. 2023. PMID: 36413413 Free PMC article.
-
[ARTICLE WITHDRAWN] Long Noncoding RNA MEG3 Inhibits Cell Proliferation and Metastasis in Chronic Myeloid Leukemia via Targeting miR-184.Oncol Res. 2018 Mar 5;26(2):297-305. doi: 10.3727/096504017X14980882803151. Epub 2017 Jun 22. Oncol Res. 2018. PMID: 28653609 Free PMC article. Retracted.
References
-
- Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. - PubMed
-
- O’Hare T, Zabriskie MS, Eiring AM, Deininger MW. Pushing the limits of targeted therapy in chronic myeloid leukaemia. Nat Rev Cancer. 2012 Aug;12(8):513–526. - PubMed
-
- Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006 Dec 7;355(23):2408–2417. - PubMed
-
- Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010 Jun 17;362(24):2251–2259. - PubMed
-
- Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010 Jun 17;362(24):2260–2270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

