Cyclin-Dependent Kinase Inhibitor AT7519 as a Potential Drug for MYCN-Dependent Neuroblastoma
- PMID: 26202950
- PMCID: PMC4645454
- DOI: 10.1158/1078-0432.CCR-15-0313
Cyclin-Dependent Kinase Inhibitor AT7519 as a Potential Drug for MYCN-Dependent Neuroblastoma
Abstract
Purpose: MYCN-dependent neuroblastomas have low cure rates with current multimodal treatment regimens and novel therapeutic drugs are therefore urgently needed. In previous preclinical studies, we have shown that targeted inhibition of cyclin-dependent kinase 2 (CDK2) resulted in specific killing of MYCN-amplified neuroblastoma cells. This study describes the in vivo preclinical evaluation of the CDK inhibitor AT7519.
Experimental design: Preclinical drug testing was performed using a panel of MYCN-amplified and MYCN single copy neuroblastoma cell lines and different MYCN-dependent mouse models of neuroblastoma.
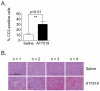
Results: AT7519 killed MYCN-amplified neuroblastoma cell lines more potently than MYCN single copy cell lines with a median LC50 value of 1.7 compared to 8.1 μmol/L (P = 0.0053) and a significantly stronger induction of apoptosis. Preclinical studies in female NMRI homozygous (nu/nu) mice with neuroblastoma patient-derived MYCN-amplified AMC711T xenografts revealed dose-dependent growth inhibition, which correlated with intratumoral AT7519 levels. CDK2 target inhibition by AT7519 was confirmed by significant reductions in levels of phosphorylated retinoblastoma (p-Rb) and nucleophosmin (p-NPM). AT7519 treatment of Th-MYCN transgenic mice resulted in improved survival and clinically significant tumor regression (average tumor size reduction of 86% at day 7 after treatment initiation). The improved efficacy of AT7519 observed in Th-MYCN mice correlated with higher tumor exposure to the drug.
Conclusions: This study strongly suggests that AT7519 is a promising drug for the treatment of high-risk neuroblastoma patients with MYCN amplification.
©2015 American Association for Cancer Research.
Figures
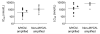

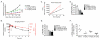

Similar articles
-
Inactivation of CDK2 is synthetically lethal to MYCN over-expressing cancer cells.Proc Natl Acad Sci U S A. 2009 Aug 4;106(31):12968-73. doi: 10.1073/pnas.0901418106. Epub 2009 Jun 12. Proc Natl Acad Sci U S A. 2009. PMID: 19525400 Free PMC article.
-
The aurora kinase inhibitor CCT137690 downregulates MYCN and sensitizes MYCN-amplified neuroblastoma in vivo.Mol Cancer Ther. 2011 Nov;10(11):2115-23. doi: 10.1158/1535-7163.MCT-11-0333. Epub 2011 Sep 1. Mol Cancer Ther. 2011. PMID: 21885865 Free PMC article.
-
Targeting ornithine decarboxylase impairs development of MYCN-amplified neuroblastoma.Cancer Res. 2009 Jan 15;69(2):547-53. doi: 10.1158/0008-5472.CAN-08-2968. Cancer Res. 2009. PMID: 19147568 Free PMC article.
-
MDM2 as MYCN transcriptional target: implications for neuroblastoma pathogenesis.Cancer Lett. 2005 Oct 18;228(1-2):21-7. doi: 10.1016/j.canlet.2005.01.050. Cancer Lett. 2005. PMID: 15927364 Review.
-
Accelerating drug development for neuroblastoma: Summary of the Second Neuroblastoma Drug Development Strategy forum from Innovative Therapies for Children with Cancer and International Society of Paediatric Oncology Europe Neuroblastoma.Eur J Cancer. 2020 Sep;136:52-68. doi: 10.1016/j.ejca.2020.05.010. Epub 2020 Jul 9. Eur J Cancer. 2020. PMID: 32653773 Review.
Cited by
-
Inflammation Resolution and the Induction of Granulocyte Apoptosis by Cyclin-Dependent Kinase Inhibitor Drugs.Front Pharmacol. 2019 Feb 19;10:55. doi: 10.3389/fphar.2019.00055. eCollection 2019. Front Pharmacol. 2019. PMID: 30886578 Free PMC article. Review.
-
High efficacy of the BCL-2 inhibitor ABT199 (venetoclax) in BCL-2 high-expressing neuroblastoma cell lines and xenografts and rational for combination with MCL-1 inhibition.Oncotarget. 2016 May 10;7(19):27946-58. doi: 10.18632/oncotarget.8547. Oncotarget. 2016. PMID: 27056887 Free PMC article.
-
3-chymotrypsin-like protease in SARS-CoV-2.Biosci Rep. 2024 Aug 28;44(8):BSR20231395. doi: 10.1042/BSR20231395. Biosci Rep. 2024. PMID: 39036877 Free PMC article. Review.
-
The HDAC6 inhibitor C1A modulates autophagy substrates in diverse cancer cells and induces cell death.Br J Cancer. 2018 Nov;119(10):1278-1287. doi: 10.1038/s41416-018-0232-5. Epub 2018 Oct 15. Br J Cancer. 2018. PMID: 30318510 Free PMC article.
-
Genome-Informed Targeted Therapy for Osteosarcoma.Cancer Discov. 2019 Jan;9(1):46-63. doi: 10.1158/2159-8290.CD-17-1152. Epub 2018 Sep 28. Cancer Discov. 2019. PMID: 30266815 Free PMC article.
References
-
- Kaelin WG., Jr. The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5(9):689–98. - PubMed
-
- Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

