Multiple Gastrointestinal Polyps in Patients Treated with BRAF Inhibitors
- PMID: 26202952
- PMCID: PMC4668213
- DOI: 10.1158/1078-0432.CCR-15-0469
Multiple Gastrointestinal Polyps in Patients Treated with BRAF Inhibitors
Abstract
Purpose: BRAF inhibitors (BRAFi) extend survival in BRAF-mutant melanoma but can promote the growth of Ras-mutant neoplasms. This study determined if gastrointestinal polyps found in BRAFi-treated patients harbored Ras mutations.
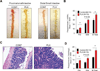
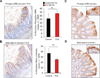
Experimental design: Colonic and gastric polyps were identified and resected from BRAFi-treated melanoma patients. Next-generation sequencing (NGS) was performed on polyps. The ability of BRAFi to promote polyp formation was functionally characterized in Apc Min(+/-) mice. MAPK and β-catenin pathway activity was assessed by immunohistochemistry in mouse and human polyps.
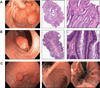
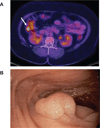
Results: Fourteen patients treated with BRAFi underwent endoscopy to assess for polyps. Seven out of 7 patients >40 years of age and treated for >2 years were found to have colonic tubular adenomas with 4 out of the 7 patients having 5 or more polyps. One patient presented with bleeding from hyperplastic gastric polyps that recurred 6 months after BRAFi rechallenge. NGS performed on polyps found no mutations in MAPK pathway genes, but found APC mutations in all tubular adenomas. A significant increase in the number of polyps was observed in BRAFi-treated compared with control-treated Apc Min(+/-) mice (20.8 ± 9.2 vs 12.8 ± 0.1; P = 0.016). No polyps were observed in BRAFi-treated wild-type mice.
Conclusions: BRAFi may increase the risk of developing hyperplastic gastric polyps and colonic adenomatous polyps. Due to the risk of gastrointestinal bleeding and the possibility of malignant transformation, further studies are needed to determine whether or not endoscopic surveillance should be recommended for patients treated with BRAFi.
©2015 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest Disclosure: RKA received grant funding from Genentech for this project. GRG is an employee of Genentech. There are no other conflicts of interest to disclose.
Figures
Similar articles
-
Phase I Dose-Escalation and -Expansion Study of the BRAF Inhibitor Encorafenib (LGX818) in Metastatic BRAF-Mutant Melanoma.Clin Cancer Res. 2017 Sep 15;23(18):5339-5348. doi: 10.1158/1078-0432.CCR-16-2923. Epub 2017 Jun 13. Clin Cancer Res. 2017. PMID: 28611198 Clinical Trial.
-
Comparative profile of cutaneous adverse events: BRAF/MEK inhibitor combination therapy versus BRAF monotherapy in melanoma.J Am Acad Dermatol. 2014 Dec;71(6):1102-1109.e1. doi: 10.1016/j.jaad.2014.09.002. Epub 2014 Oct 16. J Am Acad Dermatol. 2014. PMID: 25440439 Free PMC article.
-
PD-L1 status does not predict the outcome of BRAF inhibitor therapy in metastatic melanoma.Eur J Cancer. 2018 Jan;88:67-76. doi: 10.1016/j.ejca.2017.10.026. Epub 2017 Nov 28. Eur J Cancer. 2018. PMID: 29195116
-
[New treatment options for metastatic melanoma].Dtsch Med Wochenschr. 2014 Jul;139(28-29):1462-7. doi: 10.1055/s-0034-1370155. Epub 2014 Jul 1. Dtsch Med Wochenschr. 2014. PMID: 24983194 Review. German. No abstract available.
-
Vemurafenib for the treatment of BRAF mutant metastatic melanoma.Future Oncol. 2015;11(4):579-89. doi: 10.2217/fon.14.252. Future Oncol. 2015. PMID: 25686114 Review.
Cited by
-
Molecular targeted therapy of BRAF-mutant colorectal cancer.Ther Adv Med Oncol. 2019 Jun 18;11:1758835919856494. doi: 10.1177/1758835919856494. eCollection 2019. Ther Adv Med Oncol. 2019. PMID: 31244912 Free PMC article. Review.
-
PLX8394, a new generation BRAF inhibitor, selectively inhibits BRAF in colonic adenocarcinoma cells and prevents paradoxical MAPK pathway activation.Mol Cancer. 2017 Jun 28;16(1):112. doi: 10.1186/s12943-017-0684-x. Mol Cancer. 2017. PMID: 28659148 Free PMC article.
-
Targeting the Mutational Landscape of Bystander Cells: Drug-Promoted Blood Cancer From High-Prevalence Pre-neoplasias in Patients on BRAF Inhibitors.Front Oncol. 2020 Sep 11;10:540030. doi: 10.3389/fonc.2020.540030. eCollection 2020. Front Oncol. 2020. PMID: 33042833 Free PMC article.
-
BRAF inhibitor treatment of melanoma causing colonic polyps: An alternative hypothesis.World J Gastroenterol. 2017 May 7;23(17):3022-3029. doi: 10.3748/wjg.v23.i17.3022. World J Gastroenterol. 2017. PMID: 28533659 Free PMC article.
-
Management of Treatment-Related Adverse Events with Agents Targeting the MAPK Pathway in Patients with Metastatic Melanoma.Oncologist. 2017 Jul;22(7):823-833. doi: 10.1634/theoncologist.2016-0456. Epub 2017 May 18. Oncologist. 2017. PMID: 28526719 Free PMC article. Review.
References
-
- Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. - PubMed
-
- Puzanov I AR, McArthur GA, Flaherty KT,Chapman PB,Sosman JA, Ribas A, Shackleton M, Hwug PCB, Nolop K, Lin PS, Kim KB. Long-term outcome in BRAFV600E melanoma patients treated with vemurafenib: Patterns of disease progression and clinical management of limited progression. European Journal of Cancer. 2015 May 13; pii: S0959-8049(15)00346-9. doi:10.1016. - PMC - PubMed
-
- Ascierto PA, Minor D, Ribas A, Lebbe C, O'Hagan A, Arya N, et al. Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J Clin Oncol. 2013;31:3205–3211. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

