An integrative role for the superior colliculus in selecting targets for movements
- PMID: 26203103
- PMCID: PMC4595612
- DOI: 10.1152/jn.00262.2015
An integrative role for the superior colliculus in selecting targets for movements
Abstract
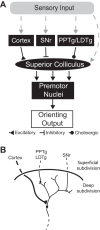
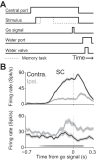
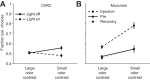
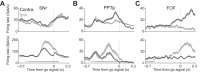
A fundamental goal of systems neuroscience is to understand the neural mechanisms underlying decision making. The midbrain superior colliculus (SC) is known to be central to the selection of one among many potential spatial targets for movements, which represents an important form of decision making that is tractable to rigorous experimental investigation. In this review, we first discuss data from mammalian models-including primates, cats, and rodents-that inform our understanding of how neural activity in the SC underlies the selection of targets for movements. We then examine the anatomy and physiology of inputs to the SC from three key regions that are themselves implicated in motor decisions-the basal ganglia, parabrachial region, and neocortex-and discuss how they may influence SC activity related to target selection. Finally, we discuss the potential for methodological advances to further our understanding of the neural bases of target selection. Our overarching goal is to synthesize what is known about how the SC and its inputs act together to mediate the selection of targets for movements, to highlight open questions about this process, and to spur future studies addressing these questions.
Keywords: decision making; laterodorsal tegmental nucleus; motor planning; pedunculopontine tegmental nucleus; substantia nigra.
Copyright © 2015 the American Physiological Society.
Figures
Similar articles
-
Functional coupling between target selection and acquisition in the superior colliculus.J Neurophysiol. 2021 Nov 1;126(5):1524-1535. doi: 10.1152/jn.00263.2021. Epub 2021 Sep 22. J Neurophysiol. 2021. PMID: 34550032 Free PMC article.
-
Spatial representations in the superior colliculus are modulated by competition among targets.Neuroscience. 2019 Jun 1;408:191-203. doi: 10.1016/j.neuroscience.2019.04.002. Epub 2019 Apr 11. Neuroscience. 2019. PMID: 30981865 Free PMC article.
-
Pathways from the Superior Colliculus to the Basal Ganglia.Curr Neuropharmacol. 2024;22(9):1431-1453. doi: 10.2174/1570159X21666230911102118. Curr Neuropharmacol. 2024. PMID: 37702174 Free PMC article. Review.
-
Inhibitory neurons in the superior colliculus mediate selection of spatially-directed movements.Commun Biol. 2021 Jun 11;4(1):719. doi: 10.1038/s42003-021-02248-1. Commun Biol. 2021. PMID: 34117346 Free PMC article.
-
Target selection and the superior colliculus: goals, choices and hypotheses.Vision Res. 2004 Jun;44(12):1445-51. doi: 10.1016/j.visres.2004.01.005. Vision Res. 2004. PMID: 15066403 Review.
Cited by
-
A collicular map for touch-guided tongue control.Nature. 2025 Jan;637(8048):1143-1151. doi: 10.1038/s41586-024-08339-3. Epub 2025 Jan 1. Nature. 2025. PMID: 39743594
-
A Descending Circuit Derived From the Superior Colliculus Modulates Vibrissal Movements.Front Neural Circuits. 2018 Nov 22;12:100. doi: 10.3389/fncir.2018.00100. eCollection 2018. Front Neural Circuits. 2018. PMID: 30524249 Free PMC article.
-
Monosynaptic inputs to specific cell types of the intermediate and deep layers of the superior colliculus.J Comp Neurol. 2020 Sep 1;528(13):2254-2268. doi: 10.1002/cne.24888. Epub 2020 Feb 29. J Comp Neurol. 2020. PMID: 32080842 Free PMC article.
-
Association Cortex Is Essential to Reverse Hemianopia by Multisensory Training.Cereb Cortex. 2021 Oct 1;31(11):5015-5023. doi: 10.1093/cercor/bhab138. Cereb Cortex. 2021. PMID: 34056645 Free PMC article.
-
Functional coupling between target selection and acquisition in the superior colliculus.J Neurophysiol. 2021 Nov 1;126(5):1524-1535. doi: 10.1152/jn.00263.2021. Epub 2021 Sep 22. J Neurophysiol. 2021. PMID: 34550032 Free PMC article.
References
-
- Aizawa H, Kobayashi Y, Yamamoto M, Isa T. Injection of nicotine into the superior colliculus facilitates occurrence of express saccades in monkeys. J Neurophysiol 82: 1642–1646, 1999. - PubMed
-
- Aronoff R, Matyas F, Mateo C, Ciron C, Schneider B, Petersen CC. Long-range connectivity of mouse primary somatosensory barrel cortex. Eur J Neurosci 31: 2221–2233, 2010. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

