Functional Oxygen Sensitivity of Astrocytes
- PMID: 26203141
- PMCID: PMC4510287
- DOI: 10.1523/JNEUROSCI.0045-15.2015
Functional Oxygen Sensitivity of Astrocytes
Abstract
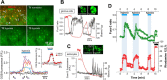
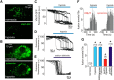
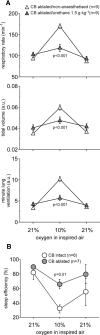
In terrestrial mammals, the oxygen storage capacity of the CNS is limited, and neuronal function is rapidly impaired if oxygen supply is interrupted even for a short period of time. However, oxygen tension monitored by the peripheral (arterial) chemoreceptors is not sensitive to regional CNS differences in partial pressure of oxygen (PO2 ) that reflect variable levels of neuronal activity or local tissue hypoxia, pointing to the necessity of a functional brain oxygen sensor. This experimental animal (rats and mice) study shows that astrocytes, the most numerous brain glial cells, are sensitive to physiological changes in PO2 . Astrocytes respond to decreases in PO2 a few millimeters of mercury below normal brain oxygenation with elevations in intracellular calcium ([Ca(2+)]i). The hypoxia sensor of astrocytes resides in the mitochondria in which oxygen is consumed. Physiological decrease in PO2 inhibits astroglial mitochondrial respiration, leading to mitochondrial depolarization, production of free radicals, lipid peroxidation, activation of phospholipase C, IP3 receptors, and release of Ca(2+) from the intracellular stores. Hypoxia-induced [Ca(2+)]i increases in astrocytes trigger fusion of vesicular compartments containing ATP. Blockade of astrocytic signaling by overexpression of ATP-degrading enzymes or targeted astrocyte-specific expression of tetanus toxin light chain (to interfere with vesicular release mechanisms) within the brainstem respiratory rhythm-generating circuits reveals the fundamental physiological role of astroglial oxygen sensitivity; in low-oxygen conditions (environmental hypoxia), this mechanism increases breathing activity even in the absence of peripheral chemoreceptor oxygen sensing. These results demonstrate that astrocytes are functionally specialized CNS oxygen sensors tuned for rapid detection of physiological changes in brain oxygenation. Significance statement: Most, if not all, animal cells possess mechanisms that allow them to detect decreases in oxygen availability leading to slow-timescale, adaptive changes in gene expression and cell physiology. To date, only two types of mammalian cells have been demonstrated to be specialized for rapid functional oxygen sensing: glomus cells of the carotid body (peripheral respiratory chemoreceptors) that stimulate breathing when oxygenation of the arterial blood decreases; and pulmonary arterial smooth muscle cells responsible for hypoxic pulmonary vasoconstriction to limit perfusion of poorly ventilated regions of the lungs. Results of the present study suggest that there is another specialized oxygen-sensitive cell type in the body, the astrocyte, that is tuned for rapid detection of physiological changes in brain oxygenation.
Keywords: astrocyte; glia; hypoxia; oxygen; respiration.
Copyright © 2015 the authors 0270-6474/15/3510461-14$15.00/0.
Figures




References
-
- Bisgard GE, Forster HV, Klein JP. Recovery of peripheral chemoreceptor function after denervation in ponies. J Appl Physiol Respir Environ Exerc Physiol. 1980;49:964–970. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
