Finerenone Impedes Aldosterone-dependent Nuclear Import of the Mineralocorticoid Receptor and Prevents Genomic Recruitment of Steroid Receptor Coactivator-1
- PMID: 26203193
- PMCID: PMC4571943
- DOI: 10.1074/jbc.M115.657957
Finerenone Impedes Aldosterone-dependent Nuclear Import of the Mineralocorticoid Receptor and Prevents Genomic Recruitment of Steroid Receptor Coactivator-1
Abstract
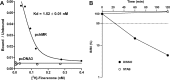
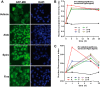
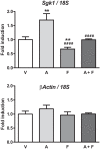
Aldosterone regulates sodium homeostasis by activating the mineralocorticoid receptor (MR), a member of the nuclear receptor superfamily. Hyperaldosteronism leads todeleterious effects on the kidney, blood vessels, and heart. Although steroidal antagonists such as spironolactone and eplerenone are clinically useful for the treatment of cardiovascular diseases, they are associated with several side effects. Finerenone, a novel nonsteroidal MR antagonist, is presently being evaluated in two clinical phase IIb trials. Here, we characterized the molecular mechanisms of action of finerenone and spironolactone at several key steps of the MR signaling pathway. Molecular modeling and mutagenesis approaches allowed identification of Ser-810 and Ala-773 as key residues for the high MR selectivity of finerenone. Moreover, we showed that, in contrast to spironolactone, which activates the S810L mutant MR responsible for a severe form of early onset hypertension, finerenone displays strict antagonistic properties. Aldosterone-dependent phosphorylation and degradation of MR are inhibited by both finerenone and spironolactone. However, automated quantification of MR subcellular distribution demonstrated that finerenone delays aldosterone-induced nuclear accumulation of MR more efficiently than spironolactone. Finally, chromatin immunoprecipitation assays revealed that, as opposed to spironolactone, finerenone inhibits MR, steroid receptor coactivator-1, and RNA polymerase II binding at the regulatory sequence of the SCNN1A gene and also remarkably reduces basal MR and steroid receptor coactivator-1 recruitment, unraveling a specific and unrecognized inactivating mechanism on MR signaling. Overall, our data demonstrate that the highly potent and selective MR antagonist finerenone specifically impairs several critical steps of the MR signaling pathway and therefore represents a promising new generation MR antagonist.
Keywords: aldosterone; antagonist; cardiovascular disease; drug action; heart; inhibition mechanism; inhibitor; mechanism of action; mineralocorticoid receptor; steroid hormone receptor.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Funder J. W. (2010) Aldosterone and mineralocorticoid receptors: past, present, and future. Endocrinology 151, 5098–5102 - PubMed
-
- Tomaschitz A., Pilz S., Ritz E., Obermayer-Pietsch B., Pieber T. R. (2010) Aldosterone and arterial hypertension. Nat. Rev. Endocrinol. 6, 83–93 - PubMed
-
- Rossi G. P., Bernini G., Desideri G., Fabris B., Ferri C., Giacchetti G., Letizia C., Maccario M., Mannelli M., Matterello M.-J., Montemurro D., Palumbo G., Rizzoni D., Rossi E., Pessina A. C., Mantero F., and PAPY Study Participants (2006) Renal damage in primary aldosteronism: results of the PAPY Study. Hypertension 48, 232–238 - PubMed
-
- Epstein M. (2006) Aldosterone blockade: an emerging strategy for abrogating progressive renal disease. Am. J. Med. 119, 912–919 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

