High Affinity Dopamine D3 Receptor (D3R)-Selective Antagonists Attenuate Heroin Self-Administration in Wild-Type but not D3R Knockout Mice
- PMID: 26203768
- PMCID: PMC4937837
- DOI: 10.1021/acs.jmedchem.5b00776
High Affinity Dopamine D3 Receptor (D3R)-Selective Antagonists Attenuate Heroin Self-Administration in Wild-Type but not D3R Knockout Mice
Abstract
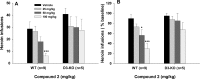
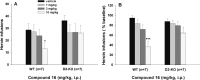
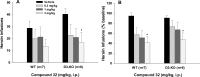
The dopamine D3 receptor (D3R) is a promising target for the development of pharmacotherapeutics to treat substance use disorders. Several D3R-selective antagonists are effective in animal models of drug abuse, especially in models of relapse. Nevertheless, poor bioavailability, metabolic instability, and/or predicted toxicity have impeded success in translating these drug candidates to clinical use. Herein, we report a series of D3R-selective 4-phenylpiperazines with improved metabolic stability. A subset of these compounds was evaluated for D3R functional efficacy and off-target binding at selected 5-HT receptor subtypes, where significant overlap in SAR with D3R has been observed. Several high affinity D3R antagonists, including compounds 16 (Ki = 0.12 nM) and 32 (Ki = 0.35 nM), showed improved metabolic stability compared to the parent compound, PG648 (6). Notably, 16 and the classic D3R antagonist SB277011A (2) were effective in reducing self-administration of heroin in wild-type but not D3R knockout mice.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

