Interactions between muscle stem cells, mesenchymal-derived cells and immune cells in muscle homeostasis, regeneration and disease
- PMID: 26203859
- PMCID: PMC4650743
- DOI: 10.1038/cddis.2015.198
Interactions between muscle stem cells, mesenchymal-derived cells and immune cells in muscle homeostasis, regeneration and disease
Abstract
Recent evidence has revealed the importance of reciprocal functional interactions between different types of mononuclear cells in coordinating the repair of injured muscles. In particular, signals released from the inflammatory infiltrate and from mesenchymal interstitial cells (also known as fibro-adipogenic progenitors (FAPs)) appear to instruct muscle stem cells (satellite cells) to break quiescence, proliferate and differentiate. Interestingly, conditions that compromise the functional integrity of this network can bias muscle repair toward pathological outcomes that are typically observed in chronic muscular disorders, that is, fibrotic and fatty muscle degeneration as well as myofiber atrophy. In this review, we will summarize the current knowledge on the regulation of this network in physiological and pathological conditions, and anticipate the potential contribution of its cellular components to relatively unexplored conditions, such as aging and physical exercise.
Figures


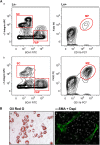
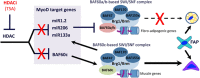

References
-
- 1Marquis K, Debigare R, Lacasse Y, LeBlanc P, Jobin J, Carrier G et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002; 166: 809–813. - PubMed
-
- 2Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008; 9: 629–635. - PubMed
-
- 3Langen RC, Gosker HR, Remels AH, Schols AM. Triggers and mechanisms of skeletal muscle wasting in chronic obstructive pulmonary disease. Int J Biochem Cell Biol 2013; 45: 2245–2256. - PubMed
-
- 5Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev 2004; 84: 209–238. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

