Condensin targets and reduces unwound DNA structures associated with transcription in mitotic chromosome condensation
- PMID: 26204128
- PMCID: PMC4525155
- DOI: 10.1038/ncomms8815
Condensin targets and reduces unwound DNA structures associated with transcription in mitotic chromosome condensation
Abstract
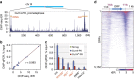
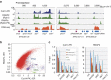
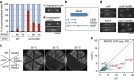
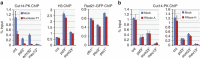
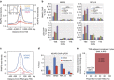
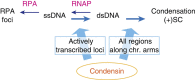
Chromosome condensation is a hallmark of mitosis in eukaryotes and is a prerequisite for faithful segregation of genetic material to daughter cells. Here we show that condensin, which is essential for assembling condensed chromosomes, helps to preclude the detrimental effects of gene transcription on mitotic condensation. ChIP-seq profiling reveals that the fission yeast condensin preferentially binds to active protein-coding genes in a transcription-dependent manner during mitosis. Pharmacological and genetic attenuation of transcription largely rescue bulk chromosome segregation defects observed in condensin mutants. We also demonstrate that condensin is associated with and reduces unwound DNA segments generated by transcription, providing a direct link between an in vitro activity of condensin and its in vivo function. The human condensin isoform condensin I also binds to unwound DNA regions at the transcription start sites of active genes, implying that our findings uncover a fundamental feature of condensin complexes.
Figures


References
-
- Koshland D. & Strunnikov A. Mitotic chromosome condensation. Annu. Rev. Cell Dev. Biol. 12, 305–333 (1996). - PubMed
-
- Belmont A. S. Mitotic chromosome structure and condensation. Curr. Opin. Cell Biol. 18, 632–638 (2006). - PubMed
-
- Baxter J. & Aragón L. A model for chromosome condensation based on the interplay between condensin and topoisomerase II. Trends Genet. 28, 110–117 (2012). - PubMed
-
- Nasmyth K. & Haering C. H. The structure and function of SMC and kleisin complexes. Annu. Rev. Biochem. 74, 595–648 (2005). - PubMed
-
- Hudson D. F., Marshall K. M. & Earnshaw W. C. Condensin: Architect of mitotic chromosomes. Chromosome Res. 17, 131–144 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

