Adjustment of Open-Loop Settings to Improve Closed-Loop Results in Type 1 Diabetes: A Multicenter Randomized Trial
- PMID: 26204135
- PMCID: PMC4596045
- DOI: 10.1210/jc.2015-2081
Adjustment of Open-Loop Settings to Improve Closed-Loop Results in Type 1 Diabetes: A Multicenter Randomized Trial
Abstract
Context: Closed-loop control (CLC) relies on an individual's open-loop insulin pump settings to initialize the system. Optimizing open-loop settings before using CLC usually requires significant time and effort.
Objective: The objective was to investigate the effects of a one-time algorithmic adjustment of basal rate and insulin to carbohydrate ratio open-loop settings on the performance of CLC.
Design: This study reports a multicenter, outpatient, randomized, crossover clinical trial.
Patients: Thirty-seven adults with type 1 diabetes were enrolled at three clinical sites.
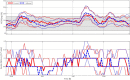
Interventions: Each subject's insulin pump settings were subject to a one-time algorithmic adjustment based on 1 week of open-loop (i.e., home care) data collection. Subjects then underwent two 27-hour periods of CLC in random order with either unchanged (control) or algorithmic adjusted basal rate and carbohydrate ratio settings (adjusted) used to initialize the zone-model predictive control artificial pancreas controller. Subject's followed their usual meal-plan and had an unannounced exercise session.
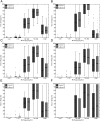
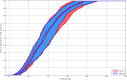
Main outcomes and measures: Time in the glucose range was 80-140 mg/dL, compared between both arms.
Results: Thirty-two subjects completed the protocol. Median time in CLC was 25.3 hours. The median time in the 80-140 mg/dl range was similar in both groups (39.7% control, 44.2% adjusted). Subjects in both arms of CLC showed minimal time spent less than 70 mg/dl (median 1.34% and 1.37%, respectively). There were no significant differences more than 140 mg/dL.
Conclusions: A one-time algorithmic adjustment of open-loop settings did not alter glucose control in a relatively short duration outpatient closed-loop study. The CLC system proved very robust and adaptable, with minimal (<2%) time spent in the hypoglycemic range in either arm.
Trial registration: ClinicalTrials.gov NCT01929798.
Figures
References
-
- Tamborlane WV, Sherwin RS, Genel M, Felig P. Reduction to normal of plasma glucose in juvenile diabetes by subcutaneous administration of insulin with a portable infusion pump. N Engl J Med. 1979;300:573–578. - PubMed
-
- Turksoy K, Quinn L, Littlejohn E, Cinar A. Multivariable adaptive identification and control for artificial pancreas systems. IEEE Trans Biomed Eng. 2014;61:883–891. - PubMed
-
- Nimri R, Phillip M. Artificial pancreas: fuzzy logic and control of glycemia. Curr Opin Endocrinol Diabetes Obes. 2014;21:251–256. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

