Combinatorial polymer matrices enhance in vitro maturation of human induced pluripotent stem cell-derived cardiomyocytes
- PMID: 26204225
- PMCID: PMC4550551
- DOI: 10.1016/j.biomaterials.2015.07.004
Combinatorial polymer matrices enhance in vitro maturation of human induced pluripotent stem cell-derived cardiomyocytes
Abstract
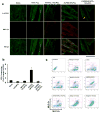
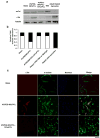
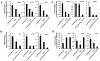
Cardiomyocytes derived from human induced pluripotent stem cells (iPSC-CMs) hold great promise for modeling human heart diseases. However, iPSC-CMs studied to date resemble immature embryonic myocytes and therefore do not adequately recapitulate native adult cardiomyocyte phenotypes. Since extracellular matrix plays an essential role in heart development and maturation in vivo, we sought to develop a synthetic culture matrix that could enhance functional maturation of iPSC-CMs in vitro. In this study, we employed a library of combinatorial polymers comprising of three functional subunits - poly-ε-caprolacton (PCL), polyethylene glycol (PEG), and carboxylated PCL (cPCL) - as synthetic substrates for culturing human iPSC-CMs. Of these, iPSC-CMs cultured on 4%PEG-96%PCL (each % indicates the corresponding molar ratio) exhibit the greatest contractility and mitochondrial function. These functional enhancements are associated with increased expression of cardiac myosin light chain-2v, cardiac troponin I and integrin alpha-7. Importantly, iPSC-CMs cultured on 4%PEG-96%PCL demonstrate troponin I (TnI) isoform switch from the fetal slow skeletal TnI (ssTnI) to the postnatal cardiac TnI (cTnI), the first report of such transition in vitro. Finally, culturing iPSC-CMs on 4%PEG-96%PCL also significantly increased expression of genes encoding intermediate filaments known to transduce integrin-mediated mechanical signals to the myofilaments. In summary, our study demonstrates that synthetic culture matrices engineered from combinatorial polymers can be utilized to promote in vitro maturation of human iPSC-CMs through the engagement of critical matrix-integrin interactions.
Keywords: Cardiomyocytes; Combinatorial polymer; Maturation; Myosin light chain-2v; Troponin I; iPSC.
Published by Elsevier Ltd.
Figures



References
-
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. - PubMed
-
- Dolnikov K, Shilkrut M, Zeevi-Levin N, Gerecht-Nir S, Amit M, Danon A, et al. Functional properties of human embryonic stem cell-derived cardiomyocytes: Intracellular Ca2+ handling and the role of sarcoplasmic reticulum in the contraction. Stem cells. 2006;24:236–45. - PubMed
-
- Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000445/TR/NCATS NIH HHS/United States
- UL1TR000445/TR/NCATS NIH HHS/United States
- R01 HL104040/HL/NHLBI NIH HHS/United States
- R01HL104040/HL/NHLBI NIH HHS/United States
- 1R01 NS078289/NS/NINDS NIH HHS/United States
- I01 BX000771/BX/BLRD VA/United States
- R01ES010563/ES/NIEHS NIH HHS/United States
- T32 GM007347/GM/NIGMS NIH HHS/United States
- R01 ES016931/ES/NIEHS NIH HHS/United States
- P50 GM115305/GM/NIGMS NIH HHS/United States
- UH2 TR000491/TR/NCATS NIH HHS/United States
- UH3 TR000491/TR/NCATS NIH HHS/United States
- R01 NS078289/NS/NINDS NIH HHS/United States
- R21 EB019509/EB/NIBIB NIH HHS/United States
- R01 ES010563/ES/NIEHS NIH HHS/United States
- R01ES016931/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

