Histamine 1 Receptor Blockade Enhances Eosinophil-Mediated Clearance of Adult Filarial Worms
- PMID: 26204515
- PMCID: PMC4512699
- DOI: 10.1371/journal.pntd.0003932
Histamine 1 Receptor Blockade Enhances Eosinophil-Mediated Clearance of Adult Filarial Worms
Erratum in
-
Correction: Histamine 1 Receptor Blockade Enhances Eosinophil-Mediated Clearance of Adult Filarial Worms.PLoS Negl Trop Dis. 2015 Aug 19;9(8):e0004034. doi: 10.1371/journal.pntd.0004034. eCollection 2015 Aug. PLoS Negl Trop Dis. 2015. PMID: 26288322 Free PMC article. No abstract available.
Abstract
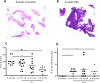
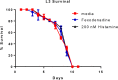
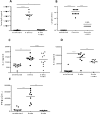
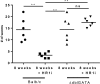
Filariae are tissue-invasive nematodes that cause diseases such as elephantiasis and river blindness. The goal of this study was to characterize the role of histamine during Litomosoides sigmodontis infection of BALB/c mice, a murine model of filariasis. Time course studies demonstrated that while expression of histidine decarboxylase mRNA increases throughout 12 weeks of infection, serum levels of histamine exhibit two peaks-one 30 minutes after primary infection and one 8 weeks later. Interestingly, mice treated with fexofenadine, a histamine receptor 1 inhibitor, demonstrated significantly reduced worm burden in infected mice compared to untreated infected controls. Although fexofenadine-treated mice had decreased antigen-specific IgE levels as well as lower splenocyte IL-5 and IFNγ production, they exhibited a greater than fourfold rise in eosinophil numbers at the tissue site where adult L. sigmodontis worms reside. Fexofenadine-mediated clearance of L. sigmodontis worms was dependent on host eosinophils, as fexofenadine did not decrease worm burdens in eosinophil-deficient dblGATA mice. These findings suggest that histamine release induced by tissue invasive helminths may aid parasite survival by diminishing eosinophilic responses. Further, these results raise the possibility that combining H1 receptor inhibitors with current anthelmintics may improve treatment efficacy for filariae and other tissue-invasive helminths.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
The immune response of inbred laboratory mice to Litomosoides sigmodontis: A route to discovery in myeloid cell biology.Parasite Immunol. 2020 Jul;42(7):e12708. doi: 10.1111/pim.12708. Epub 2020 Mar 21. Parasite Immunol. 2020. PMID: 32145033 Free PMC article. Review.
-
Susceptibility to L. sigmodontis infection is highest in animals lacking IL-4R/IL-5 compared to single knockouts of IL-4R, IL-5 or eosinophils.Parasit Vectors. 2019 May 20;12(1):248. doi: 10.1186/s13071-019-3502-z. Parasit Vectors. 2019. PMID: 31109364 Free PMC article.
-
Repeated sensitization of mice with microfilariae of Litomosoides sigmodontis induces pulmonary eosinophilia in an IL-33-dependent manner.PLoS Pathog. 2024 Mar 8;20(3):e1012071. doi: 10.1371/journal.ppat.1012071. eCollection 2024 Mar. PLoS Pathog. 2024. PMID: 38457461 Free PMC article.
-
IL-4/5 signalling plays an important role during Litomosoides sigmodontis infection, influencing both immune system regulation and tissue pathology in the thoracic cavity.Int J Parasitol. 2017 Dec;47(14):951-960. doi: 10.1016/j.ijpara.2017.06.009. Epub 2017 Aug 30. Int J Parasitol. 2017. PMID: 28859850
-
Antiallergic effects of H1-receptor antagonists.Allergy. 2000;55 Suppl 64:17-27. doi: 10.1034/j.1398-9995.2000.00803.x. Allergy. 2000. PMID: 11291777 Review.
Cited by
-
Integration of National Health Insurance claims data and animal models reveals fexofenadine as a promising repurposed drug for Parkinson's disease.J Neuroinflammation. 2024 Feb 21;21(1):53. doi: 10.1186/s12974-024-03041-7. J Neuroinflammation. 2024. PMID: 38383441 Free PMC article.
-
Eosinophil-Mediated Immune Control of Adult Filarial Nematode Infection Can Proceed in the Absence of IL-4 Receptor Signaling.J Immunol. 2020 Aug 1;205(3):731-740. doi: 10.4049/jimmunol.1901244. Epub 2020 Jun 22. J Immunol. 2020. PMID: 32571840 Free PMC article.
-
Correction: Histamine 1 Receptor Blockade Enhances Eosinophil-Mediated Clearance of Adult Filarial Worms.PLoS Negl Trop Dis. 2015 Aug 19;9(8):e0004034. doi: 10.1371/journal.pntd.0004034. eCollection 2015 Aug. PLoS Negl Trop Dis. 2015. PMID: 26288322 Free PMC article. No abstract available.
-
Eosinophils and helminth infection: protective or pathogenic?Semin Immunopathol. 2021 Jun;43(3):363-381. doi: 10.1007/s00281-021-00870-z. Epub 2021 Jun 24. Semin Immunopathol. 2021. PMID: 34165616 Review.
-
The immune response of inbred laboratory mice to Litomosoides sigmodontis: A route to discovery in myeloid cell biology.Parasite Immunol. 2020 Jul;42(7):e12708. doi: 10.1111/pim.12708. Epub 2020 Mar 21. Parasite Immunol. 2020. PMID: 32145033 Free PMC article. Review.
References
-
- Windelborg Nielsen B, Lind P, Hansen B, Nansen P, Schiotz PO (1991) Larval exoantigens from ascarid nematodes are potent inducers of histamine release from human blood basophils. Clin Exp Allergy 21: 725–732. - PubMed
-
- Jutel M, Watanabe T, Akdis M, Blaser K, Akdis CA (2002) Immune regulation by histamine. Curr Opin Immunol 14: 735–740. - PubMed
-
- Gonzalez-Munoz M, Garate T, Puente S, Subirats M, Moneo I (1999) Induction of histamine release in parasitized individuals by somatic and cuticular antigens from Onchocerca volvulus. Am J Trop Med Hyg 60: 974–979. - PubMed
-
- Ottesen EA, Neva FA, Paranjape RS, Tripathy SP, Thiruvengadam KV, et al. (1979) Specific allergic sensitsation to filarial antigens in tropical eosinophilia syndrome. Lancet 1: 1158–1161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

