Fast Simulation of Mechanical Heterogeneity in the Electrically Asynchronous Heart Using the MultiPatch Module
- PMID: 26204520
- PMCID: PMC4512705
- DOI: 10.1371/journal.pcbi.1004284
Fast Simulation of Mechanical Heterogeneity in the Electrically Asynchronous Heart Using the MultiPatch Module
Abstract
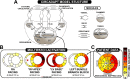
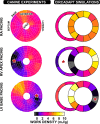
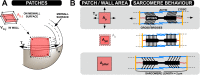
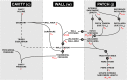
Cardiac electrical asynchrony occurs as a result of cardiac pacing or conduction disorders such as left bundle-branch block (LBBB). Electrically asynchronous activation causes myocardial contraction heterogeneity that can be detrimental for cardiac function. Computational models provide a tool for understanding pathological consequences of dyssynchronous contraction. Simulations of mechanical dyssynchrony within the heart are typically performed using the finite element method, whose computational intensity may present an obstacle to clinical deployment of patient-specific models. We present an alternative based on the CircAdapt lumped-parameter model of the heart and circulatory system, called the MultiPatch module. Cardiac walls are subdivided into an arbitrary number of patches of homogeneous tissue. Tissue properties and activation time can differ between patches. All patches within a wall share a common wall tension and curvature. Consequently, spatial location within the wall is not required to calculate deformation in a patch. We test the hypothesis that activation time is more important than tissue location for determining mechanical deformation in asynchronous hearts. We perform simulations representing an experimental study of myocardial deformation induced by ventricular pacing, and a patient with LBBB and heart failure using endocardial recordings of electrical activation, wall volumes, and end-diastolic volumes. Direct comparison between simulated and experimental strain patterns shows both qualitative and quantitative agreement between model fibre strain and experimental circumferential strain in terms of shortening and rebound stretch during ejection. Local myofibre strain in the patient simulation shows qualitative agreement with circumferential strain patterns observed in the patient using tagged MRI. We conclude that the MultiPatch module produces realistic regional deformation patterns in the asynchronous heart and that activation time is more important than tissue location within a wall for determining myocardial deformation. The CircAdapt model is therefore capable of fast and realistic simulations of dyssynchronous myocardial deformation embedded within the closed-loop cardiovascular system.
Conflict of interest statement
The authors have declared that, beyond the funding listed above, no relevant competing interests exist.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

