RUNX2 and the PI3K/AKT axis reciprocal activation as a driving force for tumor progression
- PMID: 26204939
- PMCID: PMC4513933
- DOI: 10.1186/s12943-015-0404-3
RUNX2 and the PI3K/AKT axis reciprocal activation as a driving force for tumor progression
Abstract
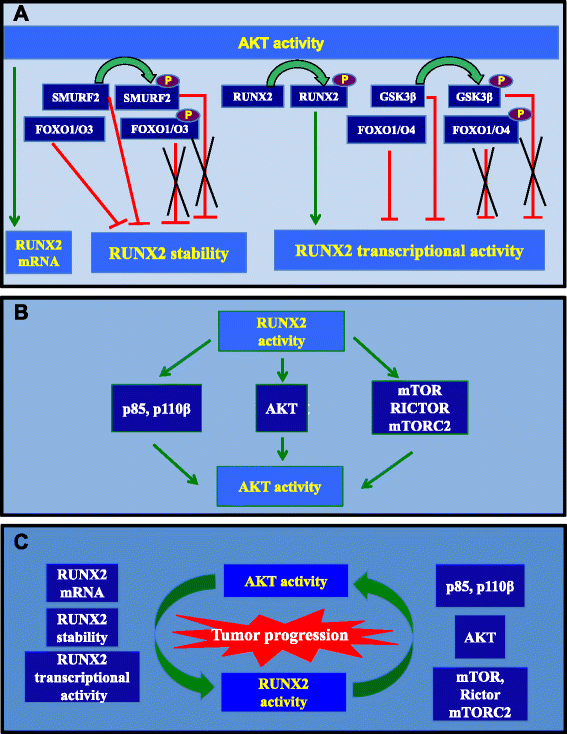
From the first reported role of the transcription factor RUNX2 in osteoblast and chondrocyte differentiation and migration to its involvement in promigratory/proinvasive behavior of breast, prostate, and thyroid cancer cells, osteosarcoma, or melanoma cells, RUNX2 currently emerges as a key player in metastasis. In this review, we address the interaction of RUNX2 with the PI3K/AKT signaling pathway, one of the critical axes controlling cancer growth and metastasis. AKT, either by directly phosphorylating/activating RUNX2 or phosphorylating/inactivating regulators of RUNX2 stability or activity, contributes to RUNX2 transcriptional activity. Reciprocally, the activation of the PI3K/AKT pathway by RUNX2 regulation of its different components has been described in non-transformed and transformed cells. This mutual activation in the context of cancer cells exhibiting constitutive AKT activation and high levels of RUNX2 might constitute a major driving force in tumor progression and aggressiveness.
Figures

References
-
- Sun SS, Zhang L, Yang J, Zhou X: Role of runt-related transcription factor 2 in signal network of tumors as an inter-mediator. Cancer Lett. 2015;361:1–7 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

