Lack of additive role of ageing in nigrostriatal neurodegeneration triggered by α-synuclein overexpression
- PMID: 26205255
- PMCID: PMC4513748
- DOI: 10.1186/s40478-015-0222-2
Lack of additive role of ageing in nigrostriatal neurodegeneration triggered by α-synuclein overexpression
Abstract
Introduction: Parkinson's disease (PD) is a progressive neurodegenerative disorder characterized by the loss of dopaminergic neurons as well as the presence of proteinaceous inclusions named Lewy bodies. α-synuclein (α-syn) is a major constituent of Lewy bodies, and the first disease-causing protein characterized in PD. Several α-syn-based animal models of PD have been developed to investigate the pathophysiology of PD, but none of them recapitulate the full picture of the disease. Ageing is the most compelling and major risk factor for developing PD but its impact on α-syn toxicity remains however unexplored. In this study, we developed and exploited a recombinant adeno-associated viral (AAV) vector of serotype 9 overexpressing mutated α-syn to elucidate the influence of ageing on the dynamics of PD-related neurodegeneration associated with α-syn pathology in different mammalian species.
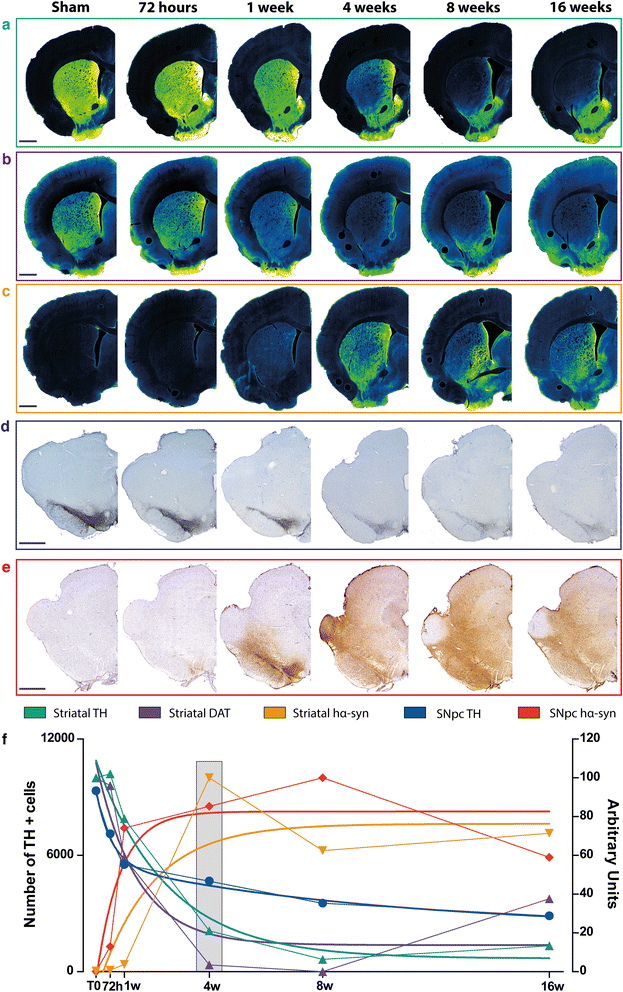
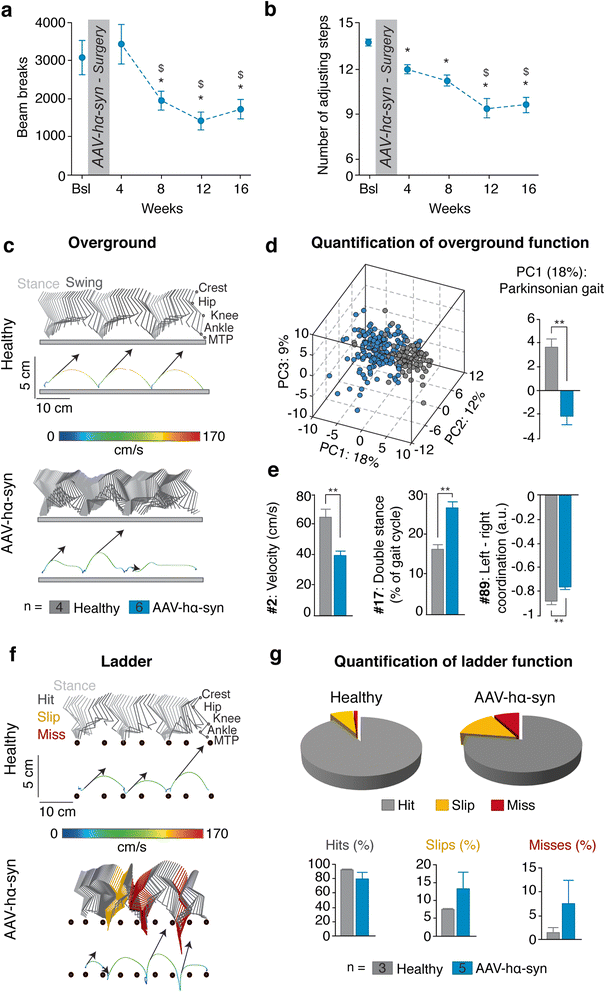
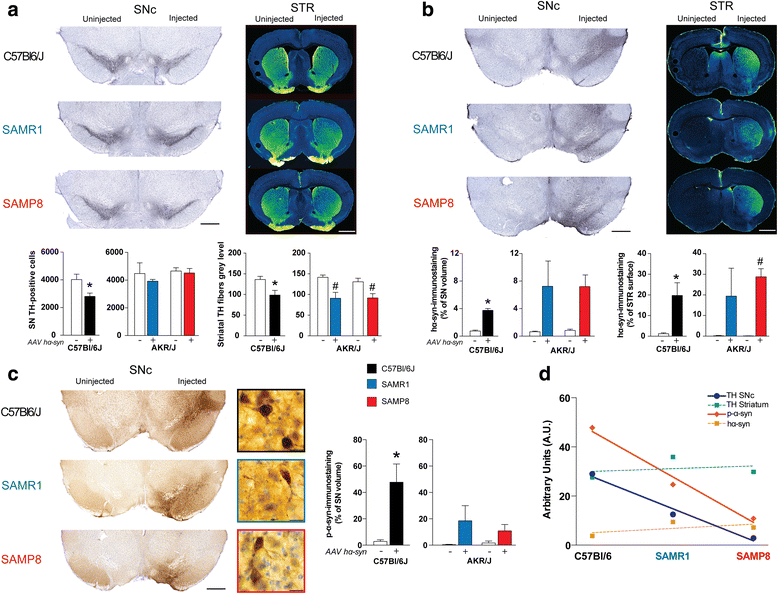
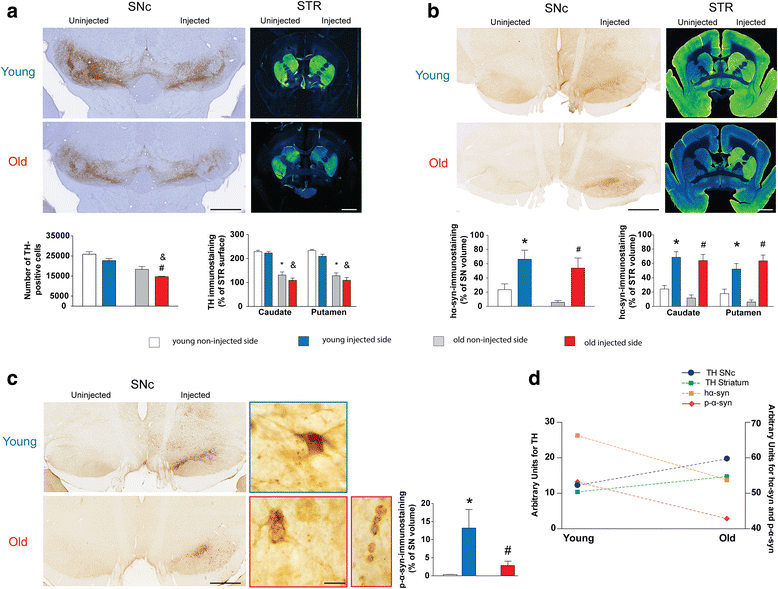
Results: Identical AAV pseudotype 2/9 vectors carrying the DNA for human mutant p.A53T α-syn were injected into the substantia nigra to induce neurodegeneration and synucleinopathy in mice, rats and monkeys. Rats were used first to validate the ability of this serotype to replicate α-syn pathology and second to investigate the relationship between the kinetics of α-syn-induced nigrostriatal degeneration and the progressive onset of motor dysfunctions, strikingly reminiscent of the impairments observed in PD patients. In mice, AAV2/9-hα-syn injection into the substantia nigra was associated with accumulation of α-syn and phosphorylated hα-syn, regardless of mouse strain. However, phenotypic mutants with either accelerated senescence or resistance to senescence did not display differential susceptibility to hα-syn overexpression. Of note, p-α-syn levels correlated with nigrostriatal degeneration in mice. In monkeys, hα-syn-induced degeneration of the nigrostriatal pathway was not affected by the age of the animals. Unlike mice, monkeys did not exhibit correlations between levels of phosphorylated α-syn and neurodegeneration.
Conclusions: In conclusion, AAV2/9-mediated hα-syn induces robust nigrostriatal neurodegeneration in mice, rats and monkeys, allowing translational comparisons among species. Ageing, however, neither exacerbated nigrostriatal neurodegeneration nor α-syn pathology per se. Our unprecedented multi-species investigation thus favours the multiple-hit hypothesis for PD wherein ageing would merely be an aggravating, additive, factor superimposed upon an independent disease process.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

