Further characterisation of transmissible spongiform encephalopathy phenotypes after inoculation of cattle with two temporally separated sources of sheep scrapie from Great Britain
- PMID: 26205536
- PMCID: PMC4618938
- DOI: 10.1186/s13104-015-1260-3
Further characterisation of transmissible spongiform encephalopathy phenotypes after inoculation of cattle with two temporally separated sources of sheep scrapie from Great Britain
Abstract
Background: The infectious agent responsible for the bovine spongiform encephalopathy (BSE) epidemic in Great Britain is a transmissible spongiform encephalopathy (TSE) strain with uniform properties but the origin of this strain remains unknown. Based on the hypothesis that classical BSE may have been caused by a TSE strain present in sheep, cattle were inoculated intracerebrally with two different pools of brains from scrapie-affected sheep sourced prior to and during the BSE epidemic to investigate resulting disease phenotypes and characterise their causal agents by transmission to rodents.
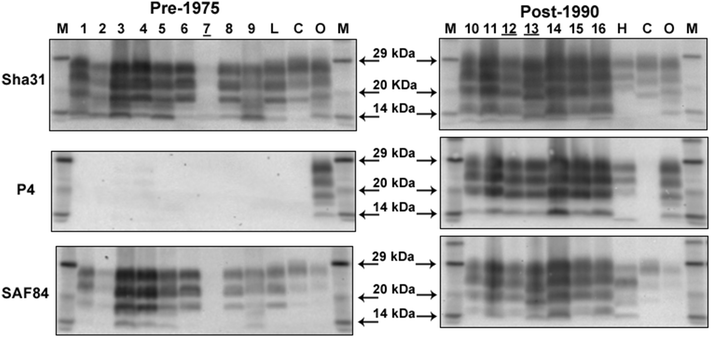
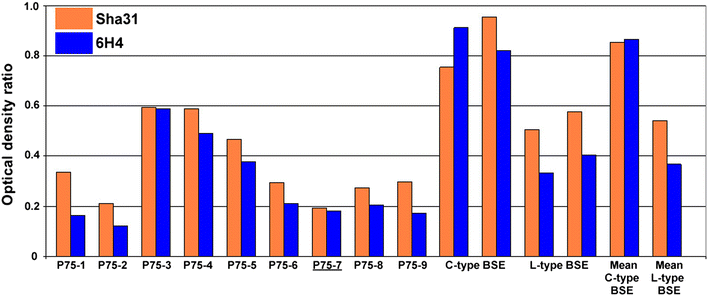
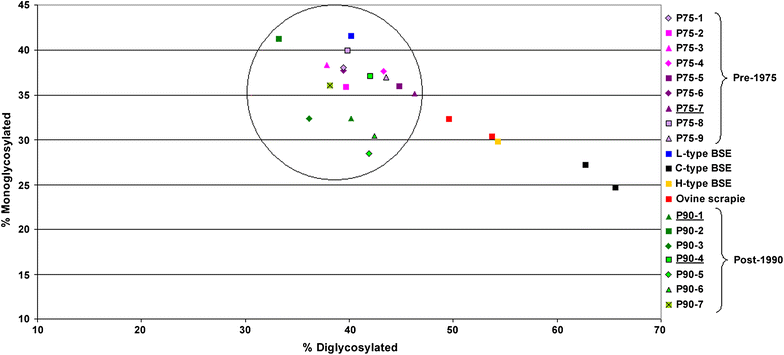
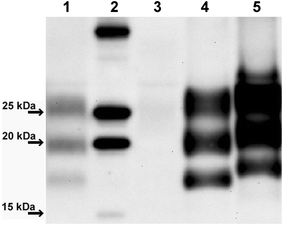
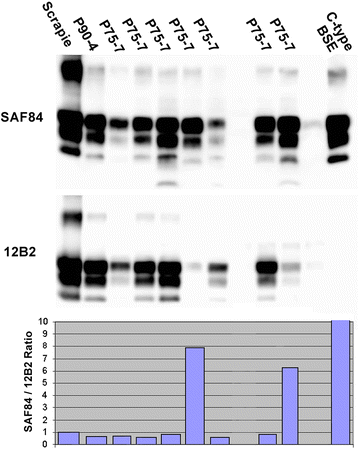
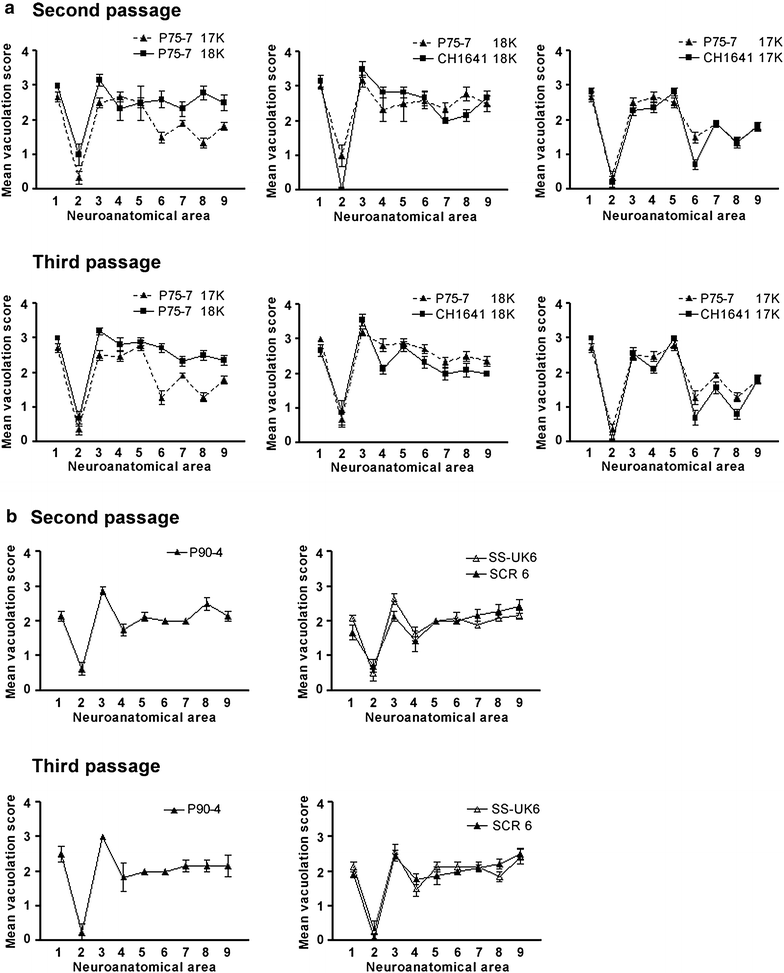
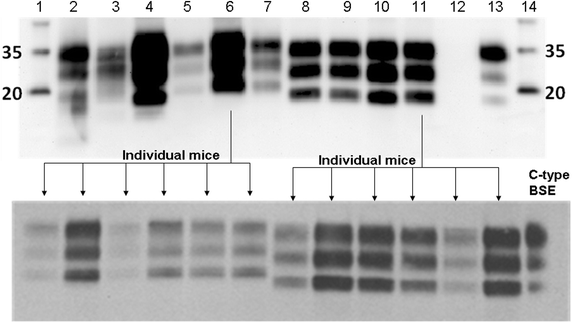
Results: As reported in 2006, intracerebral inoculation of cattle with pre-1975 and post-1990 scrapie brain pools produced two distinct disease phenotypes, which were unlike classical BSE. Subsequent to that report none of the remaining cattle, culled at 10 years post inoculation, developed a TSE. Retrospective Western immunoblot examination of the brains from TSE cases inoculated with the pre-1975 scrapie pool revealed a molecular profile similar to L-type BSE. The inoculation of transgenic mice expressing the bovine, ovine, porcine, murine or human prion protein gene and bank voles with brains from scrapie-affected cattle did not detect classical or atypical BSE strains but identified two previously characterised scrapie strains of sheep.
Conclusions: Characterisation of the causal agents of disease resulting from exposure of cattle to naturally occurring scrapie agents sourced in Great Britain did not reveal evidence of classical or atypical BSE, but did identify two distinct previously recognised strains of scrapie. Although scrapie was still recognizable upon cattle passage there were irreconcilable discrepancies between the results of biological strain typing approaches and molecular profiling methods, suggesting that the latter may not be appropriate for the identification and differentiation of atypical, particularly L-type, BSE agents from cattle experimentally infected with a potential mixture of classical scrapie strains from sheep sources.
Figures
References
-
- Wilesmith JW, Wells GA, Cranwell MP, Ryan JB. Bovine spongiform encephalopathy: epidemiological studies. Vet Rec. 1988;123:638–644. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

