Plasmodium falciparum full life cycle and Plasmodium ovale liver stages in humanized mice
- PMID: 26205537
- PMCID: PMC4525212
- DOI: 10.1038/ncomms8690
Plasmodium falciparum full life cycle and Plasmodium ovale liver stages in humanized mice
Abstract
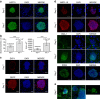
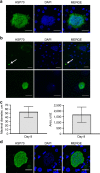
Experimental studies of Plasmodium parasites that infect humans are restricted by their host specificity. Humanized mice offer a means to overcome this and further provide the opportunity to observe the parasites in vivo. Here we improve on previous protocols to achieve efficient double engraftment of TK-NOG mice by human primary hepatocytes and red blood cells. Thus, we obtain the complete hepatic development of P. falciparum, the transition to the erythrocytic stages, their subsequent multiplication, and the appearance of mature gametocytes over an extended period of observation. Furthermore, using sporozoites derived from two P. ovale-infected patients, we show that human hepatocytes engrafted in TK-NOG mice sustain maturation of the liver stages, and the presence of late-developing schizonts indicate the eventual activation of quiescent parasites. Thus, TK-NOG mice are highly suited for in vivo observations on the Plasmodium species of humans.
Figures

References
-
- W.H.O. World Malaria Report 2013 http://www.who.int/malaria/publications/world_malaria_report_2013/en/ (2013).
-
- Galinski M. R., Meyer E. V. & Barnwell J. W. Plasmodium vivax: modern strategies to study a persistent parasite's life cycle. Adv. Parasitol. 81, 1–26 (2013). - PubMed
-
- Dembele L. et al. Persistence and activation of malaria hypnozoites in long-term primary hepatocyte cultures. Nat. Med. 20, 307–312 (2014). - PubMed
-
- Mazier D. et al. Complete development of hepatic stages of Plasmodium falciparum in vitro. Science 227, 440–442 (1985). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

