Implementation of a Molecular Tumor Board: The Impact on Treatment Decisions for 35 Patients Evaluated at Dartmouth-Hitchcock Medical Center
- PMID: 26205736
- PMCID: PMC4571816
- DOI: 10.1634/theoncologist.2015-0097
Implementation of a Molecular Tumor Board: The Impact on Treatment Decisions for 35 Patients Evaluated at Dartmouth-Hitchcock Medical Center
Abstract
Background: Although genetic profiling of tumors is a potentially powerful tool to predict drug sensitivity and resistance, its routine use has been limited because clinicians are often unfamiliar with interpretation and incorporation of the information into practice. We established a Molecular Tumor Board (MTB) to interpret individual patients' tumor genetic profiles and provide treatment recommendations.
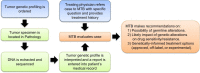
Patients and methods: DNA from tumor specimens was sequenced in a Clinical Laboratory Improvement Amendments-certified laboratory to identify coding mutations in a 50-gene panel (n = 34) or a 255-gene panel (n = 1). Cases were evaluated by a multidisciplinary MTB that included pathologists, oncologists, hematologists, basic scientists, and genetic counselors.
Results: During the first year, 35 cases were evaluated by the MTB, with 32 presented for recommendations on targeted therapies, and 3 referred for potential germline mutations. In 56.3% of cases, MTB recommended treatment with a targeted agent based on evaluation of tumor genetic profile and treatment history. Four patients (12.5%) were subsequently treated with a MTB-recommended targeted therapy; 3 of the 4 patients remain on therapy, 2 of whom experienced clinical benefit lasting >10 months.
Conclusion: For the majority of cases evaluated, the MTB was able to provide treatment recommendations based on targetable genetic alterations. The most common reasons that MTB-recommended therapy was not administered stemmed from patient preferences and genetic profiling at either very early or very late stages of disease; lack of drug access was rarely encountered. Increasing awareness of molecular profiling and targeted therapies by both clinicians and patients will improve acceptance and adherence to treatments that could significantly improve outcomes.
Implications for practice: Case evaluation by a multidisciplinary Molecular Tumor Board (MTB) is critical to benefit from individualized genetic data and maximize clinical impact. MTB recommendations shaped treatment options for the majority of cases evaluated. In the few patients treated with MTB-recommended therapy, disease outcomes were positive and support genetically informed treatment.
Keywords: Genetic profiling; Molecular tumor board; Next-generation sequencing; Precision medicine; Targeted therapy.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures



Similar articles
-
Use of dedicated gene panel sequencing using next generation sequencing to improve the personalized care of lung cancer.Oncotarget. 2016 Apr 26;7(17):24860-70. doi: 10.18632/oncotarget.8391. Oncotarget. 2016. PMID: 27027238 Free PMC article.
-
Support of a molecular tumour board by an evidence-based decision management system for precision oncology.Eur J Cancer. 2020 Mar;127:41-51. doi: 10.1016/j.ejca.2019.12.017. Epub 2020 Jan 23. Eur J Cancer. 2020. PMID: 31982633
-
Implementation of a molecular tumor board at a regional level to improve access to targeted therapy.Int J Clin Oncol. 2020 Jul;25(7):1234-1241. doi: 10.1007/s10147-020-01661-6. Epub 2020 Mar 25. Int J Clin Oncol. 2020. PMID: 32215806
-
The Personalization of Therapy: Molecular Profiling Technologies and Their Application.Semin Oncol. 2015 Dec;42(6):775-87. doi: 10.1053/j.seminoncol.2015.09.026. Epub 2015 Sep 30. Semin Oncol. 2015. PMID: 26615125 Review.
-
Molecular Tumor Boards in Clinical Practice.Trends Cancer. 2020 Sep;6(9):738-744. doi: 10.1016/j.trecan.2020.05.008. Epub 2020 Jun 6. Trends Cancer. 2020. PMID: 32517959
Cited by
-
Clinical Genotyping of Non-Small Cell Lung Cancers Using Targeted Next-Generation Sequencing: Utility of Identifying Rare and Co-mutations in Oncogenic Driver Genes.Neoplasia. 2016 Sep;18(9):577-83. doi: 10.1016/j.neo.2016.07.010. Neoplasia. 2016. PMID: 27659017 Free PMC article.
-
Key Lessons Learned from Moffitt's Molecular Tumor Board: The Clinical Genomics Action Committee Experience.Oncologist. 2017 Feb;22(2):144-151. doi: 10.1634/theoncologist.2016-0195. Epub 2017 Feb 8. Oncologist. 2017. PMID: 28179575 Free PMC article.
-
Molecular matching and treatment strategies for advanced stage lung cancer at Dartmouth-Hitchcock Medical Center: A three-year review of a Molecular Tumor Board.Pract Lab Med. 2020 Jun 12;21:e00174. doi: 10.1016/j.plabm.2020.e00174. eCollection 2020 Aug. Pract Lab Med. 2020. PMID: 32613070 Free PMC article.
-
Molecular tumour boards and molecular diagnostics for patients with cancer in the Netherlands: experiences, challenges, and aspirations.Br J Cancer. 2019 Jul;121(1):34-36. doi: 10.1038/s41416-019-0489-3. Epub 2019 May 27. Br J Cancer. 2019. PMID: 31130724 Free PMC article.
-
Laborious but Elaborate: The Benefits of Really Studying Team Dynamics.Front Psychol. 2019 Jun 28;10:1478. doi: 10.3389/fpsyg.2019.01478. eCollection 2019. Front Psychol. 2019. PMID: 31316435 Free PMC article. Review.
References
-
- Duffy MJ, O’Donovan N, Crown J. Use of molecular markers for predicting therapy response in cancer patients. Cancer Treat Rev. 2011;37:151–159. - PubMed
-
- Tsongalis GJ, Peterson JD, de Abreu FB, et al. Routine use of the Ion Torrent AmpliSeq™ Cancer Hotspot Panel for identification of clinically actionable somatic mutations. Clin Chem Lab Med. 2014;52:707–714. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

