Comparison between 5-day aprepitant and single-dose fosaprepitant meglumine for preventing nausea and vomiting induced by cisplatin-based chemotherapy
- PMID: 26206077
- PMCID: PMC4689777
- DOI: 10.1007/s00520-015-2856-9
Comparison between 5-day aprepitant and single-dose fosaprepitant meglumine for preventing nausea and vomiting induced by cisplatin-based chemotherapy
Abstract
Purpose: We aimed to compare the preventive effect of 5-day administration of aprepitant with single administration of fosaprepitant meglumine against nausea and vomiting symptoms due to highly emetogenic chemotherapy regimens comprising cisplatin (CDDP).
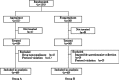
Methods: Subjects were inpatients who underwent chemotherapy for gastric cancer, esophageal cancer, lung cancer, or head and neck cancer with a regimen comprising 60 mg/m(2) or higher dose of CDDP. In this randomised, open-label, controlled study, the subjects were assigned to a group given aprepitant for 5 days or a group given a single administration of fosaprepitant meglumine. The nausea and vomiting symptoms that emerged within 7 days after the first CDDP administration were investigated with a questionnaire form; the results were compared between the two groups. Risk factors affecting nausea and vomiting symptoms were also investigated.
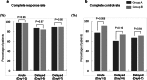
Results: Of the 101 patients enrolled, 93 patients were included (48 in the 5-day aprepitant group and 45 in the single fosaprepitant meglumine group). No significant intergroup differences in the complete response rate or the complete control rate were found over the entire period. The nausea score tended to increase from day 3 in both groups, but no significant intergroup difference was observed. Furthermore, the investigation of risk factors affecting moderate or severe nausea symptoms indicated that the fosaprepitant meglumine administration was not a risk factor.
Conclusions: Single administration of fosaprepitant meglumine was not inferior to 5-day administration of aprepitant for preventing acute and delayed nausea and vomiting symptoms occurring after administration of CDDP (60 mg/m(2) or higher).
Keywords: Aprepitant; Chemotherapy-induced nausea and vomiting; Complete response; Fosaprepitant meglumine; Highly emetogenic chemotherapy.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical