Functional role of miR-10b in tamoxifen resistance of ER-positive breast cancer cells through down-regulation of HDAC4
- PMID: 26206152
- PMCID: PMC4512090
- DOI: 10.1186/s12885-015-1561-x
Functional role of miR-10b in tamoxifen resistance of ER-positive breast cancer cells through down-regulation of HDAC4
Abstract
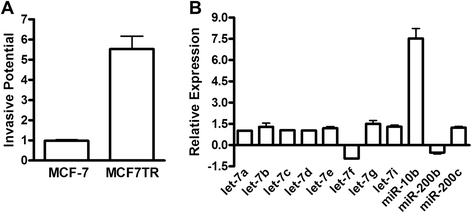
Background: For breast cancer patients diagnosed with estrogen receptor (ER)-positive tumors, treatment with tamoxifen is the gold standard. A significant number of patients, however, develop resistance to tamoxifen, and management of such tamoxifen-resistant patients is a major clinical challenge. With an eye to identify novel targets for the treatment of tamoxifen-resistant tumors, we observed that tamoxifen-resistant cells derived from ER-positive MCF-7 cells (MCF7TR) exhibit an increased expression of microRNA-10b (miR-10b). A role of miR-10b in drug-resistance of breast cancer cells has never been investigated, although its is very well known to influence invasion and metastasis.
Methods: To dileneate a role of miR-10b in tamoxifen-resistance, we over-expressed miR-10b in MCF-7 cells and down-regulated its levels in MCF7TR cells. The mechanistic role of HDAC4 in miR-10b-mediated tamoxifen resistance was studied using HDAC4 cDNA and HDAC4-specific siRNA in appropriate models.
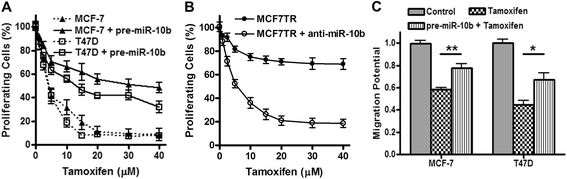
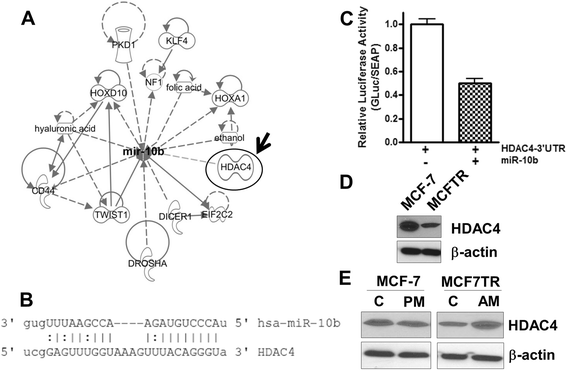
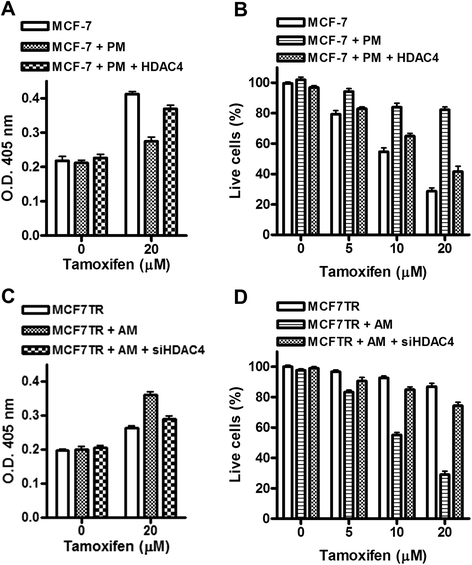
Results: Over-expression of miR-10b in ER-positive MCF-7 and T47D cells led to increased resistance to tamoxifen and an attenuation of tamoxifen-mediated inhibition of migration, whereas down-regulation of miR-10b in MCF7TR cells resulted in increased sensitivity to tamoxifen. Luciferase assays identified HDAC4 as a direct target of miR-10b. In MCF7TR cells, we observed down-regulation of HDAC4 by miR-10b. HDAC4-specific siRNA-mediated inactivation of HDAC4 in MCF-7 cells led to acquisition of tamoxifen resistance, and, moreover, reduction of HDAC4 in MCF7TR cells by HDAC4-specific siRNA transfection resulted in further enhancement of tamoxifen-resistance.
Conclusions: We propose miR-10b-HDAC4 nexus as one of the molecular mechanism of tamoxifen resistance which can potentially be expolited as a novel targeted therapeutic approach for the clinical management of tamoxifen-resistant breast cancers.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

