Activation of endplate nicotinic acetylcholine receptors by agonists
- PMID: 26206191
- PMCID: PMC4600445
- DOI: 10.1016/j.bcp.2015.06.024
Activation of endplate nicotinic acetylcholine receptors by agonists
Abstract
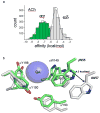
The interaction of a small molecule made in one cell with a large receptor made in another is the signature event of cell signaling. Understanding the structure and energy changes associated with agonist activation is important for engineering drugs, receptors and synapses. The nicotinic acetylcholine receptor (AChR) is a ∼300kD ion channel that binds the neurotransmitter acetylcholine (ACh) and other cholinergic agonists to elicit electrical responses in the central and peripheral nervous systems. This mini-review is in two sections. First, general concepts of skeletal muscle AChR operation are discussed in terms of energy landscapes for conformational change. Second, adult vs. fetal AChRs are compared with regard to interaction energies between ACh and agonist-site side chains, measured by single-channel electrophysiology and molecular dynamics simulations. The five aromatic residues that form the core of each agonist binding site can be divided into two working groups, a triad (led by αY190) that behaves similarly at all sites and a coupled pair (led by γW55) that has a large influence on affinity only in fetal AChRs. Each endplate AChR has 5 homologous subunits, two of α(1) and one each of β, δ, and either γ (fetal) or ϵ (adult). These nicotinic AChRs have only 2 functional agonist binding sites located in the extracellular domain, at αδ and either αγ or αϵ subunit interfaces. The receptor undergoes a reversible, global isomerization between structures called C and O. The C shape does not conduct ions and has a relatively low affinity for ACh, whereas O conducts cations and has a higher affinity. When both agonist sites are empty (filled only with water) the probability of taking on the O conformation (PO) is low, <10(-6). When ACh molecules occupy the agonist sites the C→O opening rate constant and C↔O gating equilibrium constant increase dramatically. Following a pulse of ACh at the nerve-muscle synapse, the endplate current rises rapidly to reach a peak that corresponds to PO ∼0.96.
Keywords: Acetylcholine; Allosteric; Binding; Gating; Neuromuscular; Nicotinic.
Copyright © 2015. Published by Elsevier Inc.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

