High salt primes a specific activation state of macrophages, M(Na)
- PMID: 26206316
- PMCID: PMC4528058
- DOI: 10.1038/cr.2015.87
High salt primes a specific activation state of macrophages, M(Na)
Abstract
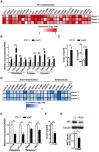
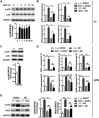
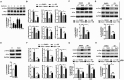
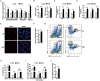
High salt is positively associated with the risk of many diseases. However, little is known about the mechanisms. Here we showed that high salt increased proinflammatory molecules, while decreased anti-inflammatory and proendocytic molecules in both human and mouse macrophages. High salt also potentiated lipopolysaccharide-induced macrophage activation and suppressed interleukin 4-induced macrophage activation. High salt induced the proinflammatory aspects by activating p38/cFos and/or Erk1/2/cFos pathways, while inhibited the anti-inflammatory and proendocytic aspects by Erk1/2/signal transducer and activator of transcription 6 pathway. Consistent with the in vitro results, high-salt diet increased proinflammatory gene expression of mouse alveolar macrophages. In mouse models of acute lung injury, high-salt diet aggravated lipopolysaccharide-induced pulmonary macrophage activation and inflammation in lungs. These results identify a novel macrophage activation state, M(Na), and high salt as a potential environmental risk factor for lung inflammation through the induction of M(Na).
Figures



Comment in
-
Sodium-activated macrophages: the salt mine expands.Cell Res. 2015 Aug;25(8):885-6. doi: 10.1038/cr.2015.91. Epub 2015 Jul 28. Cell Res. 2015. PMID: 26215700 Free PMC article.
References
-
- Pohl HR, Wheeler JS, Murray HE. Sodium and potassium in health and disease. Met Ions Life Sci. 2013;13:29–47. - PubMed
-
- O'Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–623. - PubMed
-
- Mozaffarian D, Fahimi S, Singh GM, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624–634. - PubMed
-
- Mente A, O'Donnell MJ, Rangarajan S, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med. 2014;371:601–611. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

