Rhesus rotavirus VP4 sequence-specific activation of mononuclear cells is associated with cholangiopathy in murine biliary atresia
- PMID: 26206856
- PMCID: PMC4572408
- DOI: 10.1152/ajpgi.00079.2015
Rhesus rotavirus VP4 sequence-specific activation of mononuclear cells is associated with cholangiopathy in murine biliary atresia
Abstract
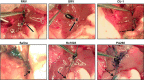
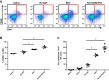
Biliary atresia (BA), a neonatal obstructive cholangiopathy, remains the most common indication for pediatric liver transplantation in the United States. In the murine model of BA, Rhesus rotavirus (RRV) VP4 surface protein determines biliary duct tropism. In this study, we investigated how VP4 governs induction of murine BA. Newborn mice were injected with 16 strains of rotavirus and observed for clinical symptoms of BA and mortality. Cholangiograms were performed to confirm bile duct obstruction. Livers and bile ducts were harvested 7 days postinfection for virus titers and histology. Flow cytometry assessed mononuclear cell activation in harvested cell populations from the liver. Cytotoxic NK cell activity was determined by the ability of NK cells to kill noninfected cholangiocytes. Of the 16 strains investigated, the 6 with the highest homology to the RRV VP4 (>87%) were capable of infecting bile ducts in vivo. Although the strain Ro1845 replicated to a titer similar to RRV in vivo, it caused no symptoms or mortality. A Ro1845 reassortant containing the RRV VP4 induced all BA symptoms, with a mortality rate of 89%. Flow cytometry revealed that NK cell activation was significantly increased in the disease-inducing strains and these NK cells demonstrated a significantly higher percentage of cytotoxicity against noninfected cholangiocytes. Rotavirus strains with >87% homology to RRV's VP4 were capable of infecting murine bile ducts in vivo. Development of murine BA was mediated by RRV VP4-specific activation of mononuclear cells, independent of viral titers.
Keywords: RRV; VP4; cholangiocytes; natural killer cells; rotavirus.
Copyright © 2015 the American Physiological Society.
Figures






Similar articles
-
Innate Immunity and Pathogenesis of Biliary Atresia.Front Immunol. 2020 Feb 25;11:329. doi: 10.3389/fimmu.2020.00329. eCollection 2020. Front Immunol. 2020. PMID: 32161597 Free PMC article. Review.
-
A Point Mutation in the Rhesus Rotavirus VP4 Protein Generated through a Rotavirus Reverse Genetics System Attenuates Biliary Atresia in the Murine Model.J Virol. 2017 Jul 12;91(15):e00510-17. doi: 10.1128/JVI.00510-17. Print 2017 Aug 1. J Virol. 2017. PMID: 28515290 Free PMC article.
-
The SRL peptide of rhesus rotavirus VP4 protein governs cholangiocyte infection and the murine model of biliary atresia.Hepatology. 2017 Apr;65(4):1278-1292. doi: 10.1002/hep.28947. Epub 2017 Jan 10. Hepatology. 2017. PMID: 27859498 Free PMC article.
-
The rhesus rotavirus gene encoding VP4 is a major determinant in the pathogenesis of biliary atresia in newborn mice.J Virol. 2011 Sep;85(17):9069-77. doi: 10.1128/JVI.02436-10. Epub 2011 Jun 22. J Virol. 2011. PMID: 21697466 Free PMC article.
-
Rotavirus and biliary atresia: can causation be proven?Curr Opin Gastroenterol. 2012 Jan;28(1):10-7. doi: 10.1097/MOG.0b013e32834c7ae4. Curr Opin Gastroenterol. 2012. PMID: 22123643 Free PMC article. Review.
Cited by
-
Innate Immunity and Pathogenesis of Biliary Atresia.Front Immunol. 2020 Feb 25;11:329. doi: 10.3389/fimmu.2020.00329. eCollection 2020. Front Immunol. 2020. PMID: 32161597 Free PMC article. Review.
-
Current Concepts of Biliary Atresia and Matrix Metalloproteinase-7: A Review of Literature.Front Med (Lausanne). 2020 Dec 21;7:617261. doi: 10.3389/fmed.2020.617261. eCollection 2020. Front Med (Lausanne). 2020. PMID: 33409288 Free PMC article. Review.
-
Specific binding sites on Rhesus rotavirus capsid protein dictate the method of endocytosis inducing the murine model of biliary atresia.Am J Physiol Gastrointest Liver Physiol. 2024 Aug 1;327(2):G267-G283. doi: 10.1152/ajpgi.00308.2023. Epub 2024 Jun 11. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 38860860 Free PMC article.
-
A Point Mutation in the Rhesus Rotavirus VP4 Protein Generated through a Rotavirus Reverse Genetics System Attenuates Biliary Atresia in the Murine Model.J Virol. 2017 Jul 12;91(15):e00510-17. doi: 10.1128/JVI.00510-17. Print 2017 Aug 1. J Virol. 2017. PMID: 28515290 Free PMC article.
-
Non-Human Primate Models of Enteric Viral Infections.Viruses. 2018 Oct 5;10(10):544. doi: 10.3390/v10100544. Viruses. 2018. PMID: 30301125 Free PMC article. Review.
References
-
- Balistreri WF, Grand R, Hoofnagle JH, Suchy FJ, Ryckman FC, Perlmutter DH, Sokol RJ. Biliary atresia: current concepts and research directions. Summary of a symposium. Hepatology 23: 1682–1692, 1996. - PubMed
-
- Bezerra JA. Potential etiologies of biliary atresia. Pediatr Transplant 9: 646–651, 2005. - PubMed
-
- Bezerra JA, Tiao G, Ryckman FC, Alonso M, Sabla GE, Shneider B, Sokol RJ, Aronow BJ. Genetic induction of proinflammatory immunity in children with biliary atresia. Lancet 360: 1653–1659, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

