Mutations in the histamine N-methyltransferase gene, HNMT, are associated with nonsyndromic autosomal recessive intellectual disability
- PMID: 26206890
- PMCID: PMC4581600
- DOI: 10.1093/hmg/ddv286
Mutations in the histamine N-methyltransferase gene, HNMT, are associated with nonsyndromic autosomal recessive intellectual disability
Abstract
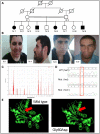
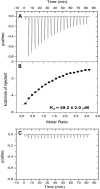
Histamine (HA) acts as a neurotransmitter in the brain, which participates in the regulation of many biological processes including inflammation, gastric acid secretion and neuromodulation. The enzyme histamine N-methyltransferase (HNMT) inactivates HA by transferring a methyl group from S-adenosyl-l-methionine to HA, and is the only well-known pathway for termination of neurotransmission actions of HA in mammalian central nervous system. We performed autozygosity mapping followed by targeted exome sequencing and identified two homozygous HNMT alterations, p.Gly60Asp and p.Leu208Pro, in patients affected with nonsyndromic autosomal recessive intellectual disability from two unrelated consanguineous families of Turkish and Kurdish ancestry, respectively. We verified the complete absence of a functional HNMT in patients using in vitro toxicology assay. Using mutant and wild-type DNA constructs as well as in silico protein modeling, we confirmed that p.Gly60Asp disrupts the enzymatic activity of the protein, and that p.Leu208Pro results in reduced protein stability, resulting in decreased HA inactivation. Our results highlight the importance of inclusion of HNMT for genetic testing of individuals presenting with intellectual disability.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures





Similar articles
-
Adult male patient with severe intellectual disability caused by a homozygous mutation in the HNMT gene.BMJ Case Rep. 2020 Dec 12;13(12):e235972. doi: 10.1136/bcr-2020-235972. BMJ Case Rep. 2020. PMID: 33310825 Free PMC article.
-
Leucine 208 in human histamine N-methyltransferase emerges as a hotspot for protein stability rationalizing the role of the L208P variant in intellectual disability.Biochim Biophys Acta Mol Basis Dis. 2017 Jan;1863(1):188-199. doi: 10.1016/j.bbadis.2016.10.005. Epub 2016 Oct 18. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 27769936
-
Human histamine N-methyltransferase pharmacogenetics: cloning and expression of kidney cDNA.Mol Pharmacol. 1994 Mar;45(3):461-8. Mol Pharmacol. 1994. PMID: 8145732
-
Histamine N-Methyltransferase in the Brain.Int J Mol Sci. 2019 Feb 10;20(3):737. doi: 10.3390/ijms20030737. Int J Mol Sci. 2019. PMID: 30744146 Free PMC article. Review.
-
The Molecular Genetics of Autosomal Recessive Nonsyndromic Intellectual Disability: a Mutational Continuum and Future Recommendations.Ann Hum Genet. 2016 Nov;80(6):342-368. doi: 10.1111/ahg.12176. Ann Hum Genet. 2016. PMID: 27870114 Review.
Cited by
-
Prevalence and Clinical Picture of Diamine Oxidase Gene Variants in Children and Adolescents with Attention Deficit Hyperactivity Disorder: A Pilot Study.J Clin Med. 2024 Mar 14;13(6):1659. doi: 10.3390/jcm13061659. J Clin Med. 2024. PMID: 38541885 Free PMC article.
-
Mapping autosomal recessive intellectual disability: combined microarray and exome sequencing identifies 26 novel candidate genes in 192 consanguineous families.Mol Psychiatry. 2018 Apr;23(4):973-984. doi: 10.1038/mp.2017.60. Epub 2017 Apr 11. Mol Psychiatry. 2018. PMID: 28397838
-
Impact of AOC1 and HNMT Variants on the Therapeutic Outcomes of a Histamine Reducing Diet in Autism Spectrum Disorder.J Mol Neurosci. 2025 Aug 12;75(3):105. doi: 10.1007/s12031-025-02399-4. J Mol Neurosci. 2025. PMID: 40794387
-
The Use of Next-Generation Sequencing for Research and Diagnostics for Intellectual Disability.Cold Spring Harb Perspect Med. 2017 Mar 1;7(3):a026864. doi: 10.1101/cshperspect.a026864. Cold Spring Harb Perspect Med. 2017. PMID: 28250017 Free PMC article. Review.
-
Adult male patient with severe intellectual disability caused by a homozygous mutation in the HNMT gene.BMJ Case Rep. 2020 Dec 12;13(12):e235972. doi: 10.1136/bcr-2020-235972. BMJ Case Rep. 2020. PMID: 33310825 Free PMC article.
References
-
- Maulik P.K., Mascarenhas M.N., Mathers C.D., Dua T., Saxena S. (2011) Prevalence of intellectual disability: a meta-analysis of population based studies. Res. Dev. Disabil., 32, 419–436. - PubMed
-
- Musante L., Ropers H.H. (2014) Genetics of recessive cognitive disorders. Trends Genet., 30, 32–39. - PubMed
-
- Loiselle J., Wollin W. (1993) Mucosal histamine elimination and its effect on acid secretion in rabbit gastric mucosa. Gastroenterology, 104, 1013–1020. - PubMed
-
- Schwartz J.C., Arrang M.M., Garbarg M., Pollaed H., Ruat M. (1991) Histaminergic transmission in the mammalian brain. Physiol. Rev., 71, 1–51. - PubMed
-
- Maslinski C. (1975) Histamine and its metabolism in mammals. Part II. Catabolism of histamine and histamine liberation. Agents Actions, 5, 183–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

