Terpenoid-Alkaloids: Their Biosynthetic Twist of Fate and Total Synthesis
- PMID: 26207071
- PMCID: PMC4508874
- DOI: 10.1002/ijch.201100005
Terpenoid-Alkaloids: Their Biosynthetic Twist of Fate and Total Synthesis
Abstract
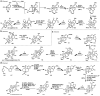
Terpenes and alkaloids are ever-growing classes of natural products that provide new molecular structures which inspire chemists and possess a broad range of biological activity. Terpenoid-alkaloids originate from the same prenyl units that construct terpene skeletons. However, during biosynthesis, a nitrogen atom (or atoms) is introduced in the form of β-aminoethanol, ethylamine, or methylamine. Nitrogen incorporation can occur either before, during, or after the cyclase phase. The outcome of this unique biosynthesis is the formation of natural products containing unprecedented structures. These complex structural motifs expose current limitations in organic chemistry, thus providing opportunities for invention. This review focuses on total syntheses of terpenoid-alkaloids and unique issues presented by this class of natural products. More specifically, it examines how these syntheses relate to the way terpenoid-alkaloids are made in Nature. Developments in chemistry that have facilitated these syntheses are emphasized, as well as chemical technology needed to conquer those that evade synthesis.
Keywords: Alkaloids; Biomimetic synthesis; C–H activation; Natural products; Total synthesis.
Figures

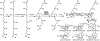

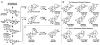
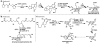



References
-
- Wang F-P, Liiang X-T. In: The Alkaloids, Chemistry and Biology. Cordell GA, editor. Vol. 59. Elsevier Science; Amsterdam: 2002. pp. 1–280. - PubMed
-
- Maimone TJ, Baran PS. Nat. Chem. Bio. 2007;3:396–407. - PubMed
- Baggaley KH, Roberts AD, Szabo LF. In: Dictionary of Alkaloids. 2nd Buckingham J, editor. CRC Press; London: 2010.
-
- Wang F-P, Chen Q-H, Liu X-Y. Nat. Prod. Rep. 2010;27:529–570. - PubMed
-
- Pfister JA, Gardner DR, Panter KE, Manners GD, Ralphs MH, Stegelmeier BL, Schoch TK. J. Nat. Toxins. 1999;8:81–94. - PubMed
-
- Ulubelen A, Meriçli AH, Meriçli F, Kilinçer N, Ferizli AG, Emekci M, Pelletier SW. Phytother. Res. 2001;15:170–171. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
