Targeting Caspase-12 to Preserve Vision in Mice With Inherited Retinal Degeneration
- PMID: 26207309
- PMCID: PMC4516015
- DOI: 10.1167/iovs.15-16924
Targeting Caspase-12 to Preserve Vision in Mice With Inherited Retinal Degeneration
Abstract
Purpose: The unfolded protein response is known to contribute to the inherited retinal pathology observed in T17M rhodopsin (T17M) mice. Recently it has been demonstrated that the endoplasmic reticulum stress-associated caspase-12 is activated during progression of retinal degeneration in different animal models. Therefore, we wanted to explore the role of caspase-12 in the mechanism of retinopathy in T17M mice and determine if inhibiting apoptosis in this way is a viable approach for halting retinal degeneration.
Methods: One, two-, and three-month-old C57BL6/J, caspase-12-/-, T17M, and T17M caspase-12-/- mice were analyzed by scotopic ERG, spectral-domain optical coherence tomography (SD-OCT), histology, quantitative (q)RT-PCR, and Western blot of retinal RNA and protein extracts. Calpain and caspase-3/7 activity assays were measured in postnatal (P) day 30 retinal extracts.
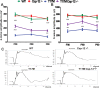
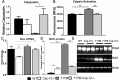
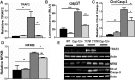
Results: Caspase-12 ablation significantly prevented a decline in the a- and b-wave ERG amplitudes in T17M mice during three months, increasing the amplitudes from 232% to 212% and from 160% to 138%, respectively, as compared to T17M retinas. The SD-OCT results and photoreceptor row counts demonstrated preservation of retinal structural integrity and postponed photoreceptor cell death. The delay in photoreceptor cell death was due to significant decreases in the activity of caspase-3/7 and calpain, which correlated with an increase in calpastatin expression.
Conclusions: We validated caspase-12 as a therapeutic target, ablation of which significantly protects T17M photoreceptors from deterioration. Although the inhibition of apoptotic activity alone was not sufficient to rescue T17M photoreceptors, in combination with other nonapoptotic targets, caspase-12 could be used to treat inherited retinopathy.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

