Phase I/II trial of capecitabine, oxaliplatin, and irinotecan in combination with bevacizumab in first line treatment of metastatic colorectal cancer
- PMID: 26207614
- PMCID: PMC4618621
- DOI: 10.1002/cam4.497
Phase I/II trial of capecitabine, oxaliplatin, and irinotecan in combination with bevacizumab in first line treatment of metastatic colorectal cancer
Abstract
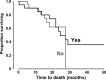
Phase III studies have demonstrated the efficacy of FOLFOXIRI regimens (5-fluorouracil/leucovorin, oxaliplatin, irinotecan) with/without bevacizumab in metastatic colorectal cancer (mCRC). Capecitabine is an orally administered fluoropyrimidine that may be used instead of 5-fluorouracil/leucovorin. We evaluated a triple-chemotherapy regimen of capecitabine, oxaliplatin, and irinotecan, plus bevacizumab in 53 patients with mCRC. A Phase I study identified the maximum tolerated dose of irinotecan as 150 mg/m². Median follow-up in a subsequent Phase II study using this dose was 28 months (74% progressed). For all patients, a complete response was achieved in 4% and a partial response in 60%; median progression-free survival (PFS) was 16 months and median overall survival (OS) was 28 months. Median PFS was longer for patients with an early treatment response (28 vs. 9 months for others; P = 0.024), or early tumor shrinkage (25 vs. 9 months for others; P = 0.006), or for patients suitable for surgical removal of metastases with curative intent (median not reached vs. 9 months for others; P = 0.001). Median OS was longer for patients with early tumor shrinkage (median not reached vs. 22 months for others; P = 0.006) or surgery (median not reached vs. 22 months for others, P = 0.002). K-ras mutations status did not influence PFS (P = 0.88) or OS (P = 0.82). Considerable Grade 3/4 toxicity was encountered (36% for diarrhea, 21% for vomiting and 17% for fatigue). In conclusion, the 3-weekly triple-chemotherapy regimen of capecitabine, oxaliplatin, and irinotecan, plus bevacizumab, was active in the first-line treatment of mCRC, although at the expense of a high level of toxicity.
Trial registration: ClinicalTrials.gov NCT01311050.
Keywords: Bevacizumab; capecitabine; irinotecan; metastatic colorectal cancer; oxaliplatin.
© 2015 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures

References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E. Forman D. Global cancer statistics. CA Cancer J. Clin. 2011;61:69. - PubMed
-
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350:2335–2342. - PubMed
-
- Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J. Clin. Oncol. 2008;26:2013–2019. - PubMed
-
- Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 2011;29:2011–2019. - PubMed
-
- Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J. Clin. Oncol. 2009;27:663–671. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

