Cell Fusion along the Anterior-Posterior Neuroaxis in Mice with Experimental Autoimmune Encephalomyelitis
- PMID: 26207625
- PMCID: PMC4514791
- DOI: 10.1371/journal.pone.0133903
Cell Fusion along the Anterior-Posterior Neuroaxis in Mice with Experimental Autoimmune Encephalomyelitis
Abstract
Background: It is well documented that bone marrow-derived cells can fuse with a diverse range of cells, including brain cells, under normal or pathological conditions. Inflammation leads to robust fusion of bone marrow-derived cells with Purkinje cells and the formation of binucleate heterokaryons in the cerebellum. Heterokaryons form through the fusion of two developmentally differential cells and as a result contain two distinct nuclei without subsequent nuclear or chromosome loss.
Aim: In the brain, fusion of bone marrow-derived cells appears to be restricted to the complex and large Purkinje cells, raising the question whether the size of the recipient cell is important for cell fusion in the central nervous system. Purkinje cells are among the largest neurons in the central nervous system and accordingly can harbor two nuclei.
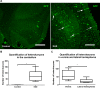
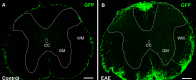
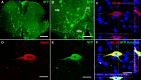
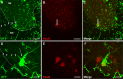
Results: Using a well-characterized model for heterokaryon formation in the cerebellum (experimental autoimmune encephalomyelitis - a mouse model of multiple sclerosis), we report for the first time that green fluorescent protein-labeled bone marrow-derived cells can fuse and form heterokaryons with spinal cord motor neurons. These spinal cord heterokaryons are predominantly located in or adjacent to an active or previously active inflammation site, demonstrating that inflammation and infiltration of immune cells are key for cell fusion in the central nervous system. While some motor neurons were found to contain two nuclei, co-expressing green fluorescent protein and the neuronal marker, neuron-specific nuclear protein, a number of small interneurons also co-expressed green fluorescent protein and the neuronal marker, neuron-specific nuclear protein. These small heterokaryons were scattered in the gray matter of the spinal cord.
Conclusion: This novel finding expands the repertoire of neurons that can form heterokaryons with bone marrow-derived cells in the central nervous system, albeit in low numbers, possibly leading to a novel therapy for spinal cord motor neurons or other neurons that are compromised in the central nervous system.
Conflict of interest statement
Figures


Similar articles
-
Aberrant cerebellar Purkinje cell function repaired in vivo by fusion with infiltrating bone marrow-derived cells.Acta Neuropathol. 2018 Jun;135(6):907-921. doi: 10.1007/s00401-018-1833-z. Epub 2018 Mar 14. Acta Neuropathol. 2018. PMID: 29541917 Free PMC article.
-
Purkinje cell fusion and binucleate heterokaryon formation in multiple sclerosis cerebellum.Brain. 2012 Oct;135(Pt 10):2962-72. doi: 10.1093/brain/aws226. Epub 2012 Sep 13. Brain. 2012. PMID: 22975392
-
Lower motor neuron loss in multiple sclerosis and experimental autoimmune encephalomyelitis.Ann Neurol. 2009 Sep;66(3):310-22. doi: 10.1002/ana.21719. Ann Neurol. 2009. PMID: 19798635
-
[PROSPECTS OF THE NUCLEAR PROTEIN NeuN APPLICATION AS AN INDEX OF FUNCTIONAL STATE OF THE VERTEBRATE NERVE CELLS].Zh Evol Biokhim Fiziol. 2015 Sep-Oct;51(5):313-23. Zh Evol Biokhim Fiziol. 2015. PMID: 26856070 Review. Russian.
-
Cell fusion in the brain: two cells forward, one cell back.Acta Neuropathol. 2014 Nov;128(5):629-38. doi: 10.1007/s00401-014-1303-1. Epub 2014 Jun 5. Acta Neuropathol. 2014. PMID: 24899142 Free PMC article. Review.
Cited by
-
Bone marrow transplantation stimulates neural repair in Friedreich's ataxia mice.Ann Neurol. 2018 Apr;83(4):779-793. doi: 10.1002/ana.25207. Ann Neurol. 2018. PMID: 29534309 Free PMC article.
-
Investigation of Endogenous Retrovirus Sequences in the Neighborhood of Genes Up-regulated in a Neuroblastoma Model after Treatment with Hypoxia-Mimetic Cobalt Chloride.Front Microbiol. 2018 Feb 21;9:287. doi: 10.3389/fmicb.2018.00287. eCollection 2018. Front Microbiol. 2018. PMID: 29515560 Free PMC article.
References
-
- Kaji K, Kudo A (2004) The mechanism of sperm-oocyte fusion in mammals. Reproduction 127: 423–429. - PubMed
-
- Huppertz B, Bartz C, Kokozidou M (2006) Trophoblast fusion: fusogenic proteins, syncytins and ADAMs, and other prerequisites for syncytial fusion. Micron 37: 509–517. - PubMed
-
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, et al. (2003) Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 425: 968–973. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

