1,8-Cineol Reduces Mucus-Production in a Novel Human Ex Vivo Model of Late Rhinosinusitis
- PMID: 26207629
- PMCID: PMC4514714
- DOI: 10.1371/journal.pone.0133040
1,8-Cineol Reduces Mucus-Production in a Novel Human Ex Vivo Model of Late Rhinosinusitis
Abstract
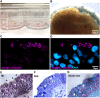
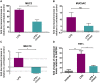
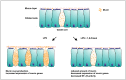
Inflammatory diseases of the respiratory system such as rhinosinusitis, chronic obstructive pulmonary disease, or bronchial asthma are strongly associated with overproduction and hypersecretion of mucus lining the epithelial airway surface. 1,8-cineol, the active ingredient of the pharmaceutical drug Soledum, is commonly applied for treating such inflammatory airway diseases. However, its potential effects on mucus overproduction still remain unclear.In the present study, we successfully established ex vivo cultures of human nasal turbinate slices to investigate the effects of 1,8-cineol on mucus hypersecretion in experimentally induced rhinosinusitis. The presence of acetyl-α-tubulin-positive cilia confirmed the integrity of the ex vivo cultured epithelium. Mucin-filled goblet cells were also detectable in nasal slice cultures, as revealed by Alcian Blue and Periodic acid-Schiff stainings. Treatment of nasal slice cultures with lipopolysaccharides mimicking bacterial infection as observed during late rhinosinusitis led to a significantly increased number of mucin-filled goblet cells. Notably, the number of mucin-filled goblet cells was found to be significantly decreased after co-treatment with 1,8-cineol. On a molecular level, real time PCR-analysis further showed 1,8-cineol to significantly reduce the expression levels of the mucin genes MUC2 and MUC19 in close association with significantly attenuated NF-κB-activity. In conclusion, we demonstrate for the first time a 1,8-cineol-dependent reduction of mucin-filled goblet cells and MUC2-gene expression associated with an attenuated NF-κB-activity in human nasal slice cultures. Our findings suggest that these effects partially account for the clinical benefits of 1,8-cineol-based therapy during rhinosinusitis. Therefore, topical application of 1,8-cineol may offer a novel therapeutic approach to reduce bacteria-induced mucus hypersecretion.
Conflict of interest statement
Figures


References
-
- Caramori G, Di Gregorio C, Carlstedt I, Casolari P, Guzzinati I, Adcock IM, et al. Mucin expression in peripheral airways of patients with chronic obstructive pulmonary disease. Histopathology. [Research Support, Non-U.S. Gov't]. 2004. November;45(5):477–84. - PubMed
-
- Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006. January;86(1):245–78. - PubMed
-
- Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. 2002. August;8(8):885–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

