Rapid, Simple and Cost-Effective Molecular Method to Differentiate the Temperature Sensitive (ts+) MS-H Vaccine Strain and Wild-Type Mycoplasma synoviae Isolates
- PMID: 26207635
- PMCID: PMC4514773
- DOI: 10.1371/journal.pone.0133554
Rapid, Simple and Cost-Effective Molecular Method to Differentiate the Temperature Sensitive (ts+) MS-H Vaccine Strain and Wild-Type Mycoplasma synoviae Isolates
Abstract
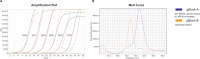
Mycoplasma synoviae infection in chickens and turkeys can cause respiratory disease, infectious synovitis and eggshell apex abnormality; thus it is an economically important pathogen. Control of M. synoviae infection comprises eradication, medication or vaccination. The differentiation of the temperature sensitive (ts+) MS-H vaccine strain from field isolates is crucial during vaccination programs. Melt-curve and agarose gel based mismatch amplification mutation assays (MAMA) are provided in the present study to distinguish between the ts+ MS-H vaccine strain, its non-temperature sensitive re-isolates and wild-type M. synoviae isolates based on the single nucleotide polymorphisms at nt367 and nt629 of the obg gene. The two melt-MAMAs and the two agarose-MAMAs clearly distinguish the ts+ MS-H vaccine strain genotype from its non-temperature sensitive re-isolate genotype and wild-type M. synoviae isolate genotype, and no cross-reactions with other Mycoplasma species infecting birds occur. The sensitivity of the melt-MAMAs and agarose-MAMAs was 103 and 104 copy numbers, respectively. The assays can be performed directly on clinical samples and they can be run simultaneously at the same annealing temperature. The assays can be performed in laboratories with limited facilities, using basic real-time PCR machine or conventional thermocycler coupled with agarose gel electrophoresis. The advantages of the described assays compared with previously used methods are simplicity, sufficient sensitivity, time and cost effectiveness and specificity.
Conflict of interest statement
Figures



References
-
- Kleven SH, Ferguson-Noel N. Mycoplasma synoviae infection In: Saif YM, Fadly AH, Glisson JR, McDougald JR, Nolan NK, Swayne DE, editors. Diseases of poultry. 12th ed. Ames: Iowa State University Press; 2008. pp. 845–856.
-
- Catania S, Bilato D, Gobbo F, Granato A, Terregino C, Iob L, et al. Treatment of eggshell abnormalities and reduced egg production caused by Mycoplasma synoviae infection. Avian Dis. 2010;54: 961–964. - PubMed
-
- Kleven SH. Control of avian mycoplasma infections in commercial poultry. Avian Dis. 2008;52: 367–374. - PubMed
-
- Marois C, Dufour-Gesbert F, Kempf I. Detection of Mycoplasma synoviae in poultry environment samples by culture and polymerase chain reaction. Vet Microbiol. 2000;73: 311–318. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

