A Biophysical Model for the Staircase Geometry of Stereocilia
- PMID: 26207893
- PMCID: PMC4514777
- DOI: 10.1371/journal.pone.0127926
A Biophysical Model for the Staircase Geometry of Stereocilia
Abstract
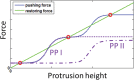
Cochlear hair cell bundles, made up of 10s to 100s of individual stereocilia, are essential for hearing, and even relatively minor structural changes, due to mutations or injuries, can result in total deafness. Consistent with its specialized role, the staircase geometry (SCG) of hair cell bundles presents one of the most striking, intricate, and precise organizations of actin-based cellular shapes. Composed of rows of actin-filled stereocilia with increasing lengths, the hair cell's staircase-shaped bundle is formed from a progenitor field of smaller, thinner, and uniformly spaced microvilli with relatively invariant lengths. While recent genetic studies have provided a significant increase in information on the multitude of stereocilia protein components, there is currently no model that integrates the basic physical forces and biochemical processes necessary to explain the emergence of the SCG. We propose such a model derived from the biophysical and biochemical characteristics of actin-based protrusions. We demonstrate that polarization of the cell's apical surface, due to the lateral polarization of the entire epithelial layer, plays a key role in promoting SCG formation. Furthermore, our model explains many distinct features of the manifestations of SCG in different species and in the presence of various deafness-associated mutations.
Conflict of interest statement
Figures

Similar articles
-
Stereocilia morphogenesis and maintenance through regulation of actin stability.Semin Cell Dev Biol. 2017 May;65:88-95. doi: 10.1016/j.semcdb.2016.08.017. Epub 2016 Aug 23. Semin Cell Dev Biol. 2017. PMID: 27565685 Free PMC article. Review.
-
Class III myosins shape the auditory hair bundles by limiting microvilli and stereocilia growth.J Cell Biol. 2016 Jan 18;212(2):231-44. doi: 10.1083/jcb.201509017. Epub 2016 Jan 11. J Cell Biol. 2016. PMID: 26754646 Free PMC article.
-
Myosin-based nucleation of actin filaments contributes to stereocilia development critical for hearing.Nat Commun. 2025 Jan 22;16(1):947. doi: 10.1038/s41467-025-55898-8. Nat Commun. 2025. PMID: 39843411 Free PMC article.
-
Correlation of expression of the actin filament-bundling protein espin with stereociliary bundle formation in the developing inner ear.J Comp Neurol. 2004 Jan 1;468(1):125-34. doi: 10.1002/cne.10944. J Comp Neurol. 2004. PMID: 14648695
-
Stereocilia Rootlets: Actin-Based Structures That Are Essential for Structural Stability of the Hair Bundle.Int J Mol Sci. 2020 Jan 3;21(1):324. doi: 10.3390/ijms21010324. Int J Mol Sci. 2020. PMID: 31947734 Free PMC article. Review.
Cited by
-
Stereocilia-staircase spacing is influenced by myosin III motors and their cargos espin-1 and espin-like.Nat Commun. 2016 Mar 1;7:10833. doi: 10.1038/ncomms10833. Nat Commun. 2016. PMID: 26926603 Free PMC article.
-
Altered Fhod3 expression involved in progressive high-frequency hearing loss via dysregulation of actin polymerization stoichiometry in the cuticular plate.PLoS Genet. 2024 Mar 18;20(3):e1011211. doi: 10.1371/journal.pgen.1011211. eCollection 2024 Mar. PLoS Genet. 2024. PMID: 38498576 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

