Evaluation of Cross-Protocol Stability of a Fully Automated Brain Multi-Atlas Parcellation Tool
- PMID: 26208327
- PMCID: PMC4514626
- DOI: 10.1371/journal.pone.0133533
Evaluation of Cross-Protocol Stability of a Fully Automated Brain Multi-Atlas Parcellation Tool
Abstract
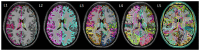
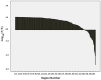
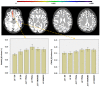
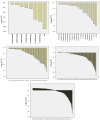
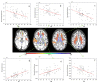
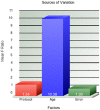
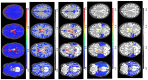
Brain parcellation tools based on multiple-atlas algorithms have recently emerged as a promising method with which to accurately define brain structures. When dealing with data from various sources, it is crucial that these tools are robust for many different imaging protocols. In this study, we tested the robustness of a multiple-atlas, likelihood fusion algorithm using Alzheimer's Disease Neuroimaging Initiative (ADNI) data with six different protocols, comprising three manufacturers and two magnetic field strengths. The entire brain was parceled into five different levels of granularity. In each level, which defines a set of brain structures, ranging from eight to 286 regions, we evaluated the variability of brain volumes related to the protocol, age, and diagnosis (healthy or Alzheimer's disease). Our results indicated that, with proper pre-processing steps, the impact of different protocols is minor compared to biological effects, such as age and pathology. A precise knowledge of the sources of data variation enables sufficient statistical power and ensures the reliability of an anatomical analysis when using this automated brain parcellation tool on datasets from various imaging protocols, such as clinical databases.
Conflict of interest statement
Figures
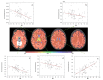
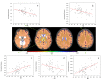
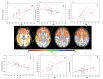
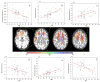
References
-
- Ashburner J, Friston KJ (2000) Voxel-based morphometry—the methods. Neuroimage 11: 805–821. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

