ICD Shock, Not Ventricular Fibrillation, Causes Elevation of High Sensitive Troponin T after Defibrillation Threshold Testing--The Prospective, Randomized, Multicentre TropShock-Trial
- PMID: 26208329
- PMCID: PMC4514854
- DOI: 10.1371/journal.pone.0131570
ICD Shock, Not Ventricular Fibrillation, Causes Elevation of High Sensitive Troponin T after Defibrillation Threshold Testing--The Prospective, Randomized, Multicentre TropShock-Trial
Abstract
Background: The placement of an implantable cardioverter defibrillator (ICD) has become routine practice to protect high risk patients from sudden cardiac death. However, implantation-related myocardial micro-damage and its relation to different implantation strategies are poorly characterized.
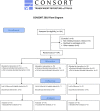
Methods: A total of 194 ICD recipients (64±12 years, 83% male, 95% primary prevention of sudden cardiac death, 35% cardiac resynchronization therapy) were randomly assigned to one of three implantation strategies: (1) ICD implantation without any defibrillation threshold (DFT) testing, (2) estimation of the DFT without arrhythmia induction (modified "upper limit of vulnerability (ULV) testing") or (3) traditional safety margin testing including ventricular arrhythmia induction. High-sensitive Troponin T (hsTnT) levels were determined prior to the implantation and 6 hours after.
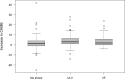
Results: All three groups showed a postoperative increase of hsTnT. The mean delta was 0.031±0.032 ng/ml for patients without DFT testing, 0.080±0.067 ng/ml for the modified ULV-testing and 0.064±0.056 ng/ml for patients with traditional safety margin testing. Delta hsTnT was significantly larger in both of the groups with intraoperative ICD testing compared to the non-testing strategy (p≤0.001 each). There was no statistical difference in delta hsTnT between the two groups with intraoperative ICD testing (p = 0.179).
Conclusion: High-sensitive Troponin T release during ICD implantation is significantly higher in patients with intraoperative ICD testing using shock applications compared to those without testing. Shock applications, with or without arrhythmia induction, did not result in a significantly different delta hsTnT. Hence, the ICD shock itself and not ventricular fibrillation seems to cause myocardial micro-damage.
Trial registration: ClinicalTrials.gov NCT01230086.
Conflict of interest statement
Figures


References
-
- Healey JS, Hohnloser SH, Glikson M, Neuzner J, Viñolas X, Mabo P, et al. The rationale and design of the Shockless IMPLant Evaluation (SIMPLE) trial: A randomized, controlled trial of defibrillation testing at the time of defibrillator implantation. Am Heart J. 2012;164:146–52. 10.1016/j.ahj.2012.05.007 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

