Amyloid-β 11C-PiB-PET imaging results from 2 randomized bapineuzumab phase 3 AD trials
- PMID: 26208959
- PMCID: PMC4553028
- DOI: 10.1212/WNL.0000000000001877
Amyloid-β 11C-PiB-PET imaging results from 2 randomized bapineuzumab phase 3 AD trials
Abstract
Objective: To evaluate the effects of bapineuzumab on brain β-amyloid (Aβ) burden using (11)C-Pittsburgh compound B ((11)C-PiB)-PET.
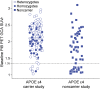
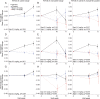
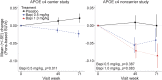
Methods: Two phase 3 clinical trials, 1 each in apolipoprotein APOE ε4 carriers and noncarriers, were conducted in patients with mild to moderate Alzheimer disease dementia. Bapineuzumab, an anti-Aβ monoclonal antibody, or placebo, was administered by IV infusion every 13 weeks for 78 weeks. PET substudies assessed change in brain fibrillar Aβ over 71 weeks using an (11)C-PiB-PET standardized uptake value ratio (SUVr) global cortical average (GCA) comprising the average SUVr from 5 cortical regions of interest with cerebellar gray matter as the reference region.
Results: A total of 115 carriers and 39 noncarriers were analyzed. The difference (δ) in mean baseline to 71 week change in (11)C-PiB-PET GCA between bapineuzumab and placebo was significant in carriers (0.5 mg/kg vs placebo δ = -0.101; p = 0.004) and in pooled analyses of both carriers and noncarriers (0.5 mg/kg vs placebo δ = -0.068; p = 0.027; 1.0 mg/kg vs placebo δ = -0.133; p = 0.028) but not in the noncarrier trial separately. Analyses by individual region of interest and in mild disease yielded findings similar to the main trial results.
Conclusions: The (11)C-PiB-PET imaging results demonstrated reduction of fibrillar Aβ accumulation in patients with Alzheimer disease treated with bapineuzumab; however, as no clinical benefit was observed, the findings are consistent with the hypotheses that bapineuzumab may not have been initiated early enough in the disease course, the doses were insufficient, or the most critical Aβ species were inadequately targeted.
Trial registration: ClinicalTrials.gov NCT00574132 NCT00575055.
© 2015 American Academy of Neurology.
Figures
Comment in
-
Comment: Yet another "disconnect" between amyloid and Alzheimer disease?Neurology. 2015 Aug 25;85(8):698. doi: 10.1212/WNL.0000000000001871. Epub 2015 Jul 24. Neurology. 2015. PMID: 26208958 No abstract available.
References
-
- Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh compound B. Ann Neurol 2004;55:306–319. - PubMed
-
- Rinne JO, Brooks DJ, Rossor MN, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol 2010;9:363–372. - PubMed
-
- Blennow K, Zetterberg H, Rinne JO, et al. Effect of immunotherapy with bapineuzumab on cerebrospinal fluid biomarker levels in patients with mild to moderate Alzheimer disease. Arch Neurol 2012;69:1002–1010. - PubMed
