Processing by MRE11 is involved in the sensitivity of subtelomeric regions to DNA double-strand breaks
- PMID: 26209132
- PMCID: PMC4652756
- DOI: 10.1093/nar/gkv714
Processing by MRE11 is involved in the sensitivity of subtelomeric regions to DNA double-strand breaks
Abstract
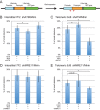
The caps on the ends of chromosomes, called telomeres, keep the ends of chromosomes from appearing as DNA double-strand breaks (DSBs) and prevent chromosome fusion. However, subtelomeric regions are sensitive to DSBs, which in normal cells is responsible for ionizing radiation-induced cell senescence and protection against oncogene-induced replication stress, but promotes chromosome instability in cancer cells that lack cell cycle checkpoints. We have previously reported that I-SceI endonuclease-induced DSBs near telomeres in a human cancer cell line are much more likely to generate large deletions and gross chromosome rearrangements (GCRs) than interstitial DSBs, but found no difference in the frequency of I-SceI-induced small deletions at interstitial and subtelomeric DSBs. We now show that inhibition of MRE11 3'-5' exonuclease activity with Mirin reduces the frequency of large deletions and GCRs at both interstitial and subtelomeric DSBs, but has little effect on the frequency of small deletions. We conclude that large deletions and GCRs are due to excessive processing of DSBs, while most small deletions occur during classical nonhomologous end joining (C-NHEJ). The sensitivity of subtelomeric regions to DSBs is therefore because they are prone to undergo excessive processing, and not because of a deficiency in C-NHEJ in subtelomeric regions.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





Similar articles
-
Differences in the recruitment of DNA repair proteins at subtelomeric and interstitial I-SceI endonuclease-induced DNA double-strand breaks.DNA Repair (Amst). 2017 Jan;49:1-8. doi: 10.1016/j.dnarep.2016.10.008. Epub 2016 Nov 5. DNA Repair (Amst). 2017. PMID: 27842255 Free PMC article.
-
The role of ATM in the deficiency in nonhomologous end-joining near telomeres in a human cancer cell line.PLoS Genet. 2013 Mar;9(3):e1003386. doi: 10.1371/journal.pgen.1003386. Epub 2013 Mar 28. PLoS Genet. 2013. PMID: 23555296 Free PMC article.
-
The DNA damage response at dysfunctional telomeres, and at interstitial and subtelomeric DNA double-strand breaks.Genes Genet Syst. 2018 Jan 20;92(3):135-152. doi: 10.1266/ggs.17-00014. Epub 2017 Nov 22. Genes Genet Syst. 2018. PMID: 29162774 Review.
-
Subtelomeric regions in mammalian cells are deficient in DNA double-strand break repair.DNA Repair (Amst). 2011 May 5;10(5):536-44. doi: 10.1016/j.dnarep.2011.03.001. Epub 2011 Apr 3. DNA Repair (Amst). 2011. PMID: 21466975 Free PMC article.
-
Ionizing radiation and genetic risks. XVII. Formation mechanisms underlying naturally occurring DNA deletions in the human genome and their potential relevance for bridging the gap between induced DNA double-strand breaks and deletions in irradiated germ cells.Mutat Res. 2013 Oct-Dec;753(2):114-130. doi: 10.1016/j.mrrev.2013.07.003. Epub 2013 Aug 12. Mutat Res. 2013. PMID: 23948232 Review.
Cited by
-
Cells and Stripes: A novel quantitative photo-manipulation technique.Sci Rep. 2016 Jan 18;6:19567. doi: 10.1038/srep19567. Sci Rep. 2016. PMID: 26777522 Free PMC article.
-
A CRISPR/Cas9-Mediated, Homology-Independent Tool Developed for Targeted Genome Integration in Yarrowia lipolytica.Appl Environ Microbiol. 2021 Feb 26;87(6):e02666-20. doi: 10.1128/AEM.02666-20. Print 2021 Feb 26. Appl Environ Microbiol. 2021. PMID: 33452022 Free PMC article.
-
Sister chromatid telomere fusions, but not NHEJ-mediated inter-chromosomal telomere fusions, occur independently of DNA ligases 3 and 4.Genome Res. 2016 May;26(5):588-600. doi: 10.1101/gr.200840.115. Epub 2016 Mar 3. Genome Res. 2016. PMID: 26941250 Free PMC article.
-
Aging and the impact of global DNA methylation, telomere shortening, and total oxidative status on sarcopenia and frailty syndrome.Immun Ageing. 2023 Nov 14;20(1):61. doi: 10.1186/s12979-023-00384-2. Immun Ageing. 2023. PMID: 37964387 Free PMC article.
-
High-throughput screen to identify compounds that prevent or target telomere loss in human cancer cells.NAR Cancer. 2022 Oct 3;4(4):zcac029. doi: 10.1093/narcan/zcac029. eCollection 2022 Dec. NAR Cancer. 2022. PMID: 36196242 Free PMC article.
References
-
- Thompson L.H. Recognition, signaling, and repair of DNA double-strand breaks produced by ionizing radiation in mammalian cells: The molecular choreography. Mutat. Res. 2012;751:158–246. - PubMed
-
- Lavin M.F. ATM and the Mre11 complex combine to recognize and signal DNA double-strand breaks. Oncogene. 2007;26:7749–7758. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

