Neutralization of Enterovirus D68 isolated from the 2014 US outbreak by commercial intravenous immune globulin products
- PMID: 26209401
- PMCID: PMC6512324
- DOI: 10.1016/j.jcv.2015.06.086
Neutralization of Enterovirus D68 isolated from the 2014 US outbreak by commercial intravenous immune globulin products
Abstract
Background: In 2014, an outbreak of Enterovirus D68 (EV-D68) was recorded as the largest in the US with cases confirmed in 49 states. Intravenous immune globulin (IVIG) has been used to treat enterovirus infections in neonates and is an accepted replacement therapy for immunodeficient patients.
Objectives: This study aimed to detect the presence of neutralizing antibodies to EV-D68 viruses from the 2014 outbreak in commercially available IVIG products.
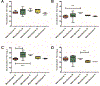
Study design: Commercially available lots of IVIG preparations were obtained from five different manufacturers (2-10 preparations per manufacturer) and tested for neutralizing antibodies against the prototype EV-D68 virus and three EV-D68 isolates representing strains circulating during the 2014 outbreak.
Results: All lots of IVIG tested were positive for EV-D68 neutralizing antibodies, with high titers ranging from 9.5log2 to 17.5log2, and with comparable median titers to all four EV-D68 viruses.
Conclusions and discussion: Amino acid sequence differences in the regions of the predicted antigenic sites on the viral capsid may explain some of the differences in neutralization among the different strains. The neutralization titers suggests that the 2014 outbreak EV-D68 viruses share some antigenic sites with the prototype virus and also present some unique antigenic sites distinct from the prototype. However, the commercial IVIG lots tested all contained high levels of neutralizing antibodies against EV-D68.
Keywords: Antibody titers; Enterovirus D68; Intravenous immunoglobulin.
Published by Elsevier B.V.
Conflict of interest statement
Conflict of Interest
None to declare.
Figures
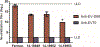

References
-
- Oberste MS, Maher K, Schnurr D, Flemister MR, Lovchik JC, Peters H, et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. The Journal of general virology 2004. September;85(Pt 9):2577–84. - PubMed
-
- Centers for Disease C, Prevention. Clusters of acute respiratory illness associated with human enterovirus 68--Asia, Europe, and United States, 2008–2010. MMWR Morbidity and mortality weekly report 2011. September 30;60(38):1301–4. - PubMed
-
- Centers for Disease C, Prevention http://www.cdc.gov/non-polio-enterovirus/outbreaks/EV-D68-outbreaks.html. 2014 3/9/2015.
-
- Arora R, Kaplan M, Nelson M. Enterovirus-specific IgG in intravenous immunoglobulin preparations. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 2011. June;106(6):544–5. - PubMed
-
- Abzug MJ, Keyserling HL, Lee ML, Levin MJ, Rotbart HA. Neonatal enterovirus infection: virology, serology, and effects of intravenous immune globulin. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 1995. May;20(5):1201–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

