Cellular and molecular mechanisms of fungal β-(1→6)-glucan in macrophages
- PMID: 26209532
- PMCID: PMC6231240
- DOI: 10.1177/1753425915595874
Cellular and molecular mechanisms of fungal β-(1→6)-glucan in macrophages
Abstract
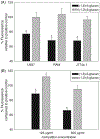
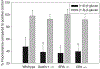
Over the last 40 yr, the majority of research on glucans has focused on β-(1→3)-glucans. Recent studies indicate that β-(1→6)-glucans may be even more potent immune modulators than β-(1→3)-glucans. Mechanisms by which β-(1→6)-glucans are recognized and modulate immunity are unknown. In this study, we examined the interaction of purified water-soluble β-(1→6)-glucans with macrophage cell lines and primary peritoneal macrophages and the cellular and molecular consequences of this interaction. Our results indicate the existence of a specific β-(1→6)-glucan receptor that internalizes the glucan ligand via a clathrin-dependent mechanism. We show that the known β-(1→3)-glucans receptors are not responsible for β-(1→6)-glucan recognition and interaction. The receptor-ligand uptake/interaction has an apparent dissociation constant (KD) of ∼ 4 µM, and was associated with phosphorylation of ERK and JNK but not IκB-α or p38. Our results indicate that macrophage interaction with β-(1→6)-glucans may lead to modulation of genes associated with anti-fungal immunity and recruitment/activation of neutrophils. In summary, we show that macrophages specifically bind and internalize β-(1→6)-glucans followed by activation of intracellular signaling and modulation of anti-fungal immune response-related gene regulation. Thus, we conclude that the interaction between innate immunity and β-(1→6)-glucans may play an important role in shaping the anti-fungal immune response.
Keywords: Fungi; glucan; innate immunity; macrophages; receptor.
© The Author(s) 2015.
Conflict of interest statement
Conflict of interest
The authors do not have any potential conflicts of interest to declare.
Figures




References
-
- Brown GD and Gordon S. Immune recognition of fungal beta-glucans. Cell Microbiol 2005; 7: 471–79. - PubMed
-
- Kankkunen P, Teirila L, Rintahaka J, et al. (1,3)-Beta-glucans activate both dectin-1 and NLRP3 inflammasome in human macrophages. J Immunol 2010; 184: 6335–6342. - PubMed
-
- Chai LY, Vonk AG, Kullberg BJ, et al. Aspergillus fumigatus cell wall components differentially modulate host TLR2 and TLR4 responses. Microbes Infect 2011; 13: 151–159. - PubMed
-
- Dennehy KM and Brown GD. The role of the beta-glucan receptor Dectin-1 in control of fungal infection. J Leukoc Biol 2007; 82: 253–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

