Zebrafish adult-derived hypothalamic neurospheres generate gonadotropin-releasing hormone (GnRH) neurons
- PMID: 26209533
- PMCID: PMC4582115
- DOI: 10.1242/bio.010447
Zebrafish adult-derived hypothalamic neurospheres generate gonadotropin-releasing hormone (GnRH) neurons
Abstract
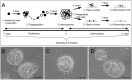
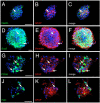
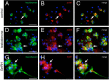
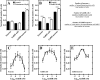
Gonadotropin-releasing hormone (GnRH) is a hypothalamic decapeptide essential for fertility in vertebrates. Human male patients lacking GnRH and treated with hormone therapy can remain fertile after cessation of treatment suggesting that new GnRH neurons can be generated during adult life. We used zebrafish to investigate the neurogenic potential of the adult hypothalamus. Previously we have characterized the development of GnRH cells in the zebrafish linking genetic pathways to the differentiation of neuromodulatory and endocrine GnRH cells in specific regions of the brain. Here, we developed a new method to obtain neural progenitors from the adult hypothalamus in vitro. Using this system, we show that neurospheres derived from the adult hypothalamus can be maintained in culture and subsequently differentiate glia and neurons. Importantly, the adult derived progenitors differentiate into neurons containing GnRH and the number of cells is increased through exposure to either testosterone or GnRH, hormones used in therapeutic treatment in humans. Finally, we show in vivo that a neurogenic niche in the hypothalamus contains GnRH positive neurons. Thus, we demonstrated for the first time that neurospheres can be derived from the hypothalamus of the adult zebrafish and that these neural progenitors are capable of producing GnRH containing neurons.
Keywords: GnRH receptors; Kallmann syndrome; Testosterone.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

