Autophagy is induced upon platelet activation and is essential for hemostasis and thrombosis
- PMID: 26209658
- PMCID: PMC4559933
- DOI: 10.1182/blood-2014-09-598722
Autophagy is induced upon platelet activation and is essential for hemostasis and thrombosis
Erratum in
- Blood. 2015 Oct 22;126(17):2072. Komatsu, Massaki [corrected to Komatsu, Masaaki]
Abstract
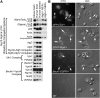
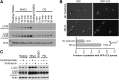
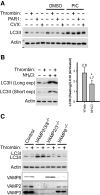
Autophagy is important for maintaining cellular homeostasis, and thus its deficiency is implicated in a broad spectrum of human diseases. Its role in platelet function has only recently been examined. Our biochemical and imaging studies demonstrate that the core autophagy machinery exists in platelets, and that autophagy is constitutively active in resting platelets. Moreover, autophagy is induced upon platelet activation, as indicated by agonist-induced loss of the autophagy marker LC3II. Additional experiments, using inhibitors of platelet activation, proteases, and lysosomal acidification, as well as platelets from knockout mouse strains, show that agonist-induced LC3II loss is a consequence of platelet signaling cascades and requires proteases, acidic compartments, and membrane fusion. To assess the physiological role of platelet autophagy, we generated a mouse strain with a megakaryocyte- and platelet-specific deletion of Atg7, an enzyme required for LC3II production. Ex vivo analysis of platelets from these mice shows modest defects in aggregation and granule cargo packaging. Although these mice have normal platelet numbers and size distributions, they exhibit a robust bleeding diathesis in the tail-bleeding assay and a prolonged occlusion time in the FeCl3-induced carotid injury model. Our results demonstrate that autophagy occurs in platelets and is important for hemostasis and thrombosis.
© 2015 by The American Society of Hematology.
Figures



References
-
- Massey AC, Kaushik S, Cuervo AM. Lysosomal chat maintains the balance. Autophagy. 2006;2(4):325–327. - PubMed
-
- Kunz JB, Schwarz H, Mayer A. Determination of four sequential stages during microautophagy in vitro. J Biol Chem. 2004;279(11):9987–9996. - PubMed
-
- Suzuki K, Ohsumi Y. Molecular machinery of autophagosome formation in yeast, Saccharomyces cerevisiae. FEBS Lett. 2007;581(11):2156–2161. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

