Roles of Thermophiles and Fungi in Bitumen Degradation in Mostly Cold Oil Sands Outcrops
- PMID: 26209669
- PMCID: PMC4561710
- DOI: 10.1128/AEM.02221-15
Roles of Thermophiles and Fungi in Bitumen Degradation in Mostly Cold Oil Sands Outcrops
Abstract
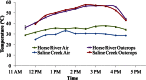
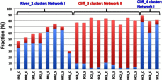
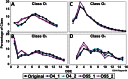
Oil sands are surface exposed in river valley outcrops in northeastern Alberta, where flat slabs (tablets) of weathered, bitumen-saturated sandstone can be retrieved from outcrop cliffs or from riverbeds. Although the average yearly surface temperature of this region is low (0.7°C), we found that the temperatures of the exposed surfaces of outcrop cliffs reached 55 to 60°C on sunny summer days, with daily maxima being 27 to 31°C. Analysis of the cooccurrence of taxa derived from pyrosequencing of 16S/18S rRNA genes indicated that an aerobic microbial network of fungi and hydrocarbon-, methane-, or acetate-oxidizing heterotrophic bacteria was present in all cliff tablets. Metagenomic analyses indicated an elevated presence of fungal cytochrome P450 monooxygenases in these samples. This network was distinct from the heterotrophic community found in riverbeds, which included fewer fungi. A subset of cliff tablets had a network of anaerobic and/or thermophilic taxa, including methanogens, Firmicutes, and Thermotogae, in the center. Long-term aerobic incubation of outcrop samples at 55°C gave a thermophilic microbial community. Analysis of residual bitumen with a Fourier transform ion cyclotron resonance mass spectrometer indicated that aerobic degradation proceeded at 55°C but not at 4°C. Little anaerobic degradation was observed. These results indicate that bitumen degradation on outcrop surfaces is a largely aerobic process with a minor anaerobic contribution and is catalyzed by a consortium of bacteria and fungi. Bitumen degradation is stimulated by periodic high temperatures on outcrop cliffs, which cause significant decreases in bitumen viscosity.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

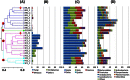
 ). The relative abundances of eukaryotic phyla (B), proteobacterial classes (C), and major phyla other than Proteobacteria (D) are also shown.
). The relative abundances of eukaryotic phyla (B), proteobacterial classes (C), and major phyla other than Proteobacteria (D) are also shown.

References
-
- Masliyah J, Zhou Z, Xu Z, Czarnecki J, Hamza H. 2004. Understanding water-based bitumen extraction from Athabasca oil sands. Can J Chem Eng 82:628–654.
-
- Adams JJ, Larter SR, Bennett B, Huang H, Westrich J, van Kruisdijk C. 2013. The dynamic interplay of oil mixing, charge timing, and biodegradation in forming the Alberta oil sands: insights from geologic modeling and biogeochemistry, p 23–102. In Hein FJ, Leckie D, Larter S, Suter J (ed), Heavy-oil and oil-sand petroleum systems in Alberta and beyond. AAPG studies in geology 64. American Association of Petroleum Geologists, Tulsa, OK.
-
- Tozer R, Choi A, Pietras J, Tanasichuk D. 2014. Athabasca oil sands: mega-trap restoration and charge timing. AAPG Bull 98:429–447. doi:10.1306/08071313039. - DOI
-
- Larter S, Head I. 2014. Oil sands and heavy oil: origin and exploitation. Elements 10:277–284. doi:10.2113/gselements.10.4.277. - DOI
-
- Evans CR, Rogers MA, Bailey NJL. 1971. Evolution and alteration of petroleum in western Canada. Chem Geol 8:147–170. doi:10.1016/0009-2541(71)90002-7. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

