Intracellular sphingosine kinase 2-derived sphingosine-1-phosphate mediates epidermal growth factor-induced ezrin-radixin-moesin phosphorylation and cancer cell invasion
- PMID: 26209696
- PMCID: PMC4608912
- DOI: 10.1096/fj.15-274340
Intracellular sphingosine kinase 2-derived sphingosine-1-phosphate mediates epidermal growth factor-induced ezrin-radixin-moesin phosphorylation and cancer cell invasion
Abstract
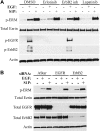
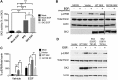
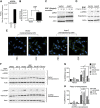
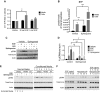
The bioactive sphingolipid sphingosine-1-phosphate (S1P) mediates cellular proliferation, mitogenesis, inflammation, and angiogenesis. These biologies are mediated through S1P binding to specific GPCRs [sphingosine-1-phosphate receptor (S1PR)1-5] and some other less well-characterized intracellular targets. Ezrin-radixin-moesin (ERM) proteins, a family of adaptor molecules linking the cortical actin cytoskeleton to the plasma membrane, are emerging as critical regulators of cancer invasion via regulation of cell morphology and motility. Recently, we identified S1P as an acute ERM activator (via phosphorylation) through its action on S1PR2. In this work, we dissect the mechanism of S1P generation downstream of epidermal growth factor (EGF) leading to ERM phosphorylation and cancer invasion. Using pharmacologic inhibitors, small interfering RNA technologies, and genetic approaches, we demonstrate that sphingosine kinase (SK)2, and not SK1, is essential and sufficient in EGF-mediated ERM phosphorylation in HeLa cells. In fact, knocking down SK2 decreased ERM activation 2.5-fold. Furthermore, we provide evidence that SK2 is necessary to mediate EGF-induced invasion. In addition, overexpressing SK2 causes a 2-fold increase in HeLa cell invasion. Surprisingly, and for the first time, we find that this event, although dependent on S1PR2 activation, does not generate and does not require extracellular S1P secretion, therefore introducing a potential novel model of autocrine/intracrine action of S1P that still involves its GPCRs. These results define new mechanistic insights for EGF-mediated invasion and novel actions of SK2, therefore setting the stage for novel targets in the treatment of growth factor-driven malignancies.
Keywords: Spns2; alkaline ceramidase 2; cell adhesion.
© FASEB.
Figures



References
-
- Hannun Y. A., Obeid L. M. (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9, 139–150 - PubMed
-
- Hannun Y. A., Bell R. M. (1989) Regulation of protein kinase C by sphingosine and lysosphingolipids. Clin. Chim. Acta 185, 333–345 - PubMed
-
- Gandy K. A., Obeid L. M. (2013) Regulation of the sphingosine kinase/sphingosine 1-phosphate pathway. Handb. Exp. Pharmacol. 216, 275–303 - PubMed
-
- Schnute M. E., McReynolds M. D., Kasten T., Yates M., Jerome G., Rains J. W., Hall T., Chrencik J., Kraus M., Cronin C. N., Saabye M., Highkin M. K., Broadus R., Ogawa S., Cukyne K., Zawadzke L. E., Peterkin V., Iyanar K., Scholten J. A., Wendling J., Fujiwara H., Nemirovskiy O., Wittwer A. J., Nagiec M. M. (2012) Modulation of cellular S1P levels with a novel, potent and specific inhibitor of sphingosine kinase-1. Biochem. J. 444, 79–88 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

