Macrophage polarization in pathology
- PMID: 26210152
- PMCID: PMC11113543
- DOI: 10.1007/s00018-015-1995-y
Macrophage polarization in pathology
Abstract
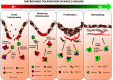
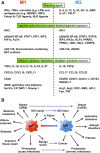
Macrophages are cells of the innate immunity constituting the mononuclear phagocyte system and endowed with remarkable different roles essential for defense mechanisms, development of tissues, and homeostasis. They derive from hematopoietic precursors and since the early steps of fetal life populate peripheral tissues, a process continuing throughout adult life. Although present essentially in every organ/tissue, macrophages are more abundant in the gastro-intestinal tract, liver, spleen, upper airways, and brain. They have phagocytic and bactericidal activity and produce inflammatory cytokines that are important to drive adaptive immune responses. Macrophage functions are settled in response to microenvironmental signals, which drive the acquisition of polarized programs, whose extremes are simplified in the M1 and M2 dichotomy. Functional skewing of monocyte/macrophage polarization occurs in physiological conditions (e.g., ontogenesis and pregnancy), as well as in pathology (allergic and chronic inflammation, tissue repair, infection, and cancer) and is now considered a key determinant of disease development and/or regression. Here, we will review evidence supporting a dynamic skewing of macrophage functions in disease, which may provide a basis for macrophage-centered therapeutic strategies.
Keywords: Disease; Inflammation; Macrophage polarization; Tissue damage; Tissue homeostasis.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

