Effect of UV-photofunctionalization on oral bacterial attachment and biofilm formation to titanium implant material
- PMID: 26210175
- PMCID: PMC4667792
- DOI: 10.1016/j.biomaterials.2015.07.030
Effect of UV-photofunctionalization on oral bacterial attachment and biofilm formation to titanium implant material
Abstract
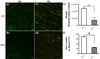
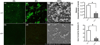
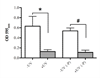
Bacterial biofilm infections remain prevalent reasons for implant failure. Dental implant placement occurs in the oral environment, which harbors a plethora of biofilm-forming bacteria. Due to its trans-mucosal placement, part of the implant structure is exposed to oral cavity and there is no effective measure to prevent bacterial attachment to implant materials. Here, we demonstrated that UV treatment of titanium immediately prior to use (photofunctionalization) affects the ability of human polymicrobial oral biofilm communities to colonize in the presence of salivary and blood components. UV-treatment of machined titanium transformed the surface from hydrophobic to superhydrophilic. UV-treated surfaces exhibited a significant reduction in bacterial attachment as well as subsequent biofilm formation compared to untreated ones, even though overall bacterial viability was not affected. The function of reducing bacterial colonization was maintained on UV-treated titanium that had been stored in a liquid environment before use. Denaturing gradient gel-electrophoresis (DGGE) and DNA sequencing analyses revealed that while bacterial community profiles appeared different between UV-treated and untreated titanium in the initial attachment phase, this difference vanished as biofilm formation progressed. Our findings confirm that UV-photofunctionalization of titanium has a strong potential to improve outcome of implant placement by creating and maintaining antimicrobial surfaces.
Keywords: Bacteria; Biofilm; Peri-implantitis; Photofunctionalization; Titanium.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures





References
-
- Pjetursson BE, Asgeirsson AG, Zwahlen M, Sailer I. Improvements in implant dentistry over the last decade: comparison of survival and complication rates in older and newer publications. Int J Oral Maxillofac Implants. 2014;29(Suppl):308–324. - PubMed
-
- Simonis P, Dufour T, Tenenbaum H. Long-term implant survival and success: a 10–16-year follow-up of non-submerged dental implants. Clin Oral Implants Res. 2010;21:772–777. - PubMed
-
- Clementini M, Rossetti PH, Penarrocha D, Micarelli C, Bonachela WC, Canullo L. Systemic risk factors for peri-implant bone loss: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2014;43:323–334. - PubMed
-
- Heitz-Mayfield LJ. Peri-implant diseases: diagnosis and risk indicators. J Clin Periodontol. 2008;35:292–304. - PubMed
-
- Aguirre-Zorzano LA, Estefania-Fresco R, Telletxea O, Bravo M. Prevalence of peri-implant inflammatory disease in patients with a history of periodontal disease who receive supportive periodontal therapy. Clin Oral Implants Res. 2014 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

