Alginate micro-encapsulation of mesenchymal stromal cells enhances modulation of the neuro-inflammatory response
- PMID: 26210574
- PMCID: PMC5928499
- DOI: 10.1016/j.jcyt.2015.05.002
Alginate micro-encapsulation of mesenchymal stromal cells enhances modulation of the neuro-inflammatory response
Abstract
Background aims: Modulation of inflammation after brain trauma is a key therapeutic goal aimed at limiting the consequences of the subsequent injury cascade. Mesenchymal stromal cells (MSCs) have been demonstrated to dynamically regulate the inflammatory environment in several tissue systems, including the central nervous system. There has been limited success, however, with the use of direct implantation of cells in the brain caused by low viability and engraftment at the injury site. To circumvent this, we encapsulated MSCs in alginate microspheres and evaluated the ability of these encapsulated MSCs to attenuate inflammation in rat organotypic hippocampal slice cultures (OHSC).
Methods: OHSC were administered lipopolysaccharide to induce inflammation and immediately co-cultured with encapsulated or monolayer human MSCs. After 24 h, culture media was assayed for the pro-inflammatory cytokine tumor necrosis factor-alpha (TNF-α) produced by OHSC, as well as MSC-produced trophic mediators.
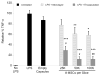
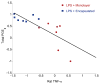
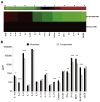
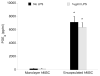
Results: Encapsulated MSCs reduced TNF-α more effectively than did monolayer MSCs. Additionally, there was a strong correlation between increased prostaglandin E2 (PGE2) and reduction of TNF-α. In contrast to monolayer MSCs, inflammatory signals were not required to stimulate PGE2 production by encapsulated MSCs. Further encapsulation-stimulated changes were revealed in a multiplex panel analyzing 27 MSC-produced cytokines and growth factors, from which additional mediators with strong correlations to TNF-α levels were identified.
Conclusions: These results suggest that alginate encapsulation of MSCs may not only provide an improved delivery vehicle for transplantation but may also enhance MSC therapeutic benefit for treating neuro-inflammation.
Keywords: co-culture; inflammation mediators; mesenchymal stromal cells; organ culture; traumatic brain injury.
Copyright © 2015. Published by Elsevier Inc.
Conflict of interest statement
Figures

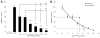


Similar articles
-
Prostaglandin E2 Produced by Alginate-Encapsulated Mesenchymal Stromal Cells Modulates the Astrocyte Inflammatory Response.Nano Life. 2017 Jun;7(2):1750005. doi: 10.1142/s1793984417500052. Nano Life. 2017. PMID: 29682085 Free PMC article.
-
Identification of IL-1β and LPS as optimal activators of monolayer and alginate-encapsulated mesenchymal stromal cell immunomodulation using design of experiments and statistical methods.Biotechnol Prog. 2015 Jul-Aug;31(4):1058-70. doi: 10.1002/btpr.2103. Epub 2015 May 28. Biotechnol Prog. 2015. PMID: 25958832 Free PMC article.
-
Encapsulation of allogeneic mesenchymal stem cells in alginate extends local presence and therapeutic function.Eur Cell Mater. 2017 Jan 30;33:43-58. doi: 10.22203/eCM.v033a04. Eur Cell Mater. 2017. PMID: 28138954
-
Alginate-liposomal construct for bupivacaine delivery and MSC function regulation.Drug Deliv Transl Res. 2018 Feb;8(1):226-238. doi: 10.1007/s13346-017-0454-8. Drug Deliv Transl Res. 2018. PMID: 29204926 Free PMC article.
-
Mesenchymal Stem/Stromal Cells Microencapsulation for Cell Therapy.Cells. 2025 Jan 21;14(3):149. doi: 10.3390/cells14030149. Cells. 2025. PMID: 39936941 Free PMC article. Review.
Cited by
-
Interleukin-10 Contributes to Therapeutic Effect of Mesenchymal Stem Cells for Acute Liver Failure via Signal Transducer and Activator of Transcription 3 Signaling Pathway.Chin Med J (Engl). 2016 Apr 20;129(8):967-75. doi: 10.4103/0366-6999.179794. Chin Med J (Engl). 2016. PMID: 27064043 Free PMC article.
-
Strategies to Potentiate Paracrine Therapeutic Efficacy of Mesenchymal Stem Cells in Inflammatory Diseases.Int J Mol Sci. 2021 Mar 25;22(7):3397. doi: 10.3390/ijms22073397. Int J Mol Sci. 2021. PMID: 33806241 Free PMC article. Review.
-
Challenges and advances in clinical applications of mesenchymal stromal cells.J Hematol Oncol. 2021 Feb 12;14(1):24. doi: 10.1186/s13045-021-01037-x. J Hematol Oncol. 2021. PMID: 33579329 Free PMC article. Review.
-
Effects of Hydrogels on Mesenchymal Stem/Stromal Cells Paracrine Activity and Extracellular Vesicles Production.J Extracell Vesicles. 2025 Mar;14(3):e70057. doi: 10.1002/jev2.70057. J Extracell Vesicles. 2025. PMID: 40091440 Free PMC article. Review.
-
Sequential modes of crosslinking tune viscoelasticity of cell-instructive hydrogels.Biomaterials. 2019 Jan;188:187-197. doi: 10.1016/j.biomaterials.2018.10.013. Epub 2018 Oct 12. Biomaterials. 2019. PMID: 30366219 Free PMC article.
References
-
- Werner C, Engelhard K. Pathophysiology of traumatic brain injury. British Journal of Anaesthesia. 2007;99:4–9. - PubMed
-
- Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62:127–36. - PubMed
-
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–11. - PubMed
-
- Mahmood A, Lu D, Qu C, Goussev A, Chopp M. Long-term recovery after bone marrow stromal cell treatment of traumatic brain injury in rats. J Neurosurg. 2006;104:272–7. - PubMed
-
- Heile AMB, Wallrapp C, Klinge PM, Samii A, Kassem M, Silverberg G, et al. Cerebral transplantation of encapsulated mesenchymal stem cells improves cellular pathology after experimental traumatic brain injury. Neurosci Lett. 2009;463:176–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

