Rapastinel (GLYX-13) has therapeutic potential for the treatment of post-traumatic stress disorder: Characterization of a NMDA receptor-mediated metaplasticity process in the medial prefrontal cortex of rats
- PMID: 26210936
- PMCID: PMC4702501
- DOI: 10.1016/j.bbr.2015.07.039
Rapastinel (GLYX-13) has therapeutic potential for the treatment of post-traumatic stress disorder: Characterization of a NMDA receptor-mediated metaplasticity process in the medial prefrontal cortex of rats
Abstract
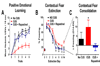
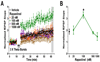
Rapastinel (GLYX-13) is a NMDA receptor modulator with glycine-site partial agonist properties. It is a robust cognitive enhancer and shows rapid and long-lasting antidepressant properties in both animal models and in humans. Contextual fear extinction (CFE) in rodents has been well characterized and used extensively as a model to study the neurobiological mechanisms of post-traumatic stress disorder (PTSD). Since CFE is NMDA receptor modulated and neural circuitry in the medial prefrontal cortex (MPFC) regulates both depression and PTSD, studies were undertaken to examine the effects of rapastinel for its therapeutic potential in PTSD and to use rapastinel as a tool to study its underlying glutamatergic mechanisms. A 21-day chronic mild unpredictable stress (CUS) rat model was used to model depression and PTSD. The effects of CUS alone compared to No CUS controls, and the effects of rapastinel (3 mg/kg IV) on CUS-treated animals were examined. The effect of rapastinel was first assessed using CUS-treated rats in three depression models, Porsolt, sucrose preference, and novelty-induced hypophagia tests, and found to produce a complete reversal of the depressive-like state in each model. Rapastinel was then assessed in a MPFC-dependent positive emotional learning paradigm and in CFE and again a reversal of the impairments induced by CUS treatment was observed. Both synaptic plasticity and metaplasticity, as measured by the induction of long-term potentiation in rat MPFC slice preparations, was found to be markedly impaired in CUS-treated animals. This impairment was reversed when CUS-treated rats were administered rapastinel and tested 24 h later. Transcriptomic analysis of MPFC mRNA expression in CUS-treated rats corroborated the link between rapastinel's behavioral effects and synaptic plasticity. A marked enrichment in both the LTP and LTD connectomes in rapastinel-treated CUS rats was observed compared to CUS-treated controls. The effects of rapastinel on depression models, PEL, and most importantly on CFE demonstrate the therapeutic potential of rapastinel for the treatment of PTSD. Moreover, rapastinel appears to elicit its therapeutic effects through a NMDA receptor-mediated, LTP-like, metaplasticity process in the MPFC.
Keywords: Depression; GLYX-13; LTP; Medial Prefrontal Cortex; NMDA Receptor.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures


References
-
- Moskal JR, Kuo AG, Weiss C, Wood PL, O'Connor Hanson A, Kelso S, et al. GLYX-13: a monoclonal antibody-derived peptide that acts as an N-methyl-D-aspartate receptor modulator. Neuropharmacology. 2005;49:1077–1087. - PubMed
-
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Archives of general psychiatry. 1998;55:626–632. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

