The Structural Role of Antibody N-Glycosylation in Receptor Interactions
- PMID: 26211613
- PMCID: PMC4558368
- DOI: 10.1016/j.str.2015.06.015
The Structural Role of Antibody N-Glycosylation in Receptor Interactions
Abstract
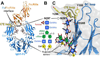
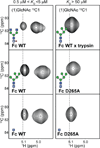
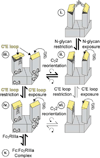
Asparagine(N)297-linked glycosylation of immunoglobulin G (IgG) Fc is required for binding to FcγRIIa, IIb, and IIIa, although it is unclear how it contributes. We found the quaternary structure of glycosylated Fc was indistinguishable from aglycosylated Fc, indicating that N-glycosylation does not maintain relative Fc Cγ2/Cγ3 domain orientation. However, the conformation of the C'E loop, which contains N297, was significantly perturbed in the aglycosylated Fc variant. The conformation of the C'E loop as measured with a range of Fc variants shows a strong correlation with FcγRIIIa affinity. These results indicate that the primary role of the IgG1 Fc N-glycan is to stabilize the C'E loop through intramolecular interactions between carbohydrate and amino acid residues, and preorganize the FcγRIIIa interface for optimal binding affinity. The features that contribute to the capacity of the IgG1 Fc N-glycan to restrict protein conformation and tune binding affinity are conserved in other antibodies including IgG2-IgG4, IgD, IgE, and IgM.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
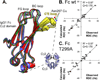



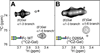
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

