Comparison of self-administered survey questionnaire responses collected using mobile apps versus other methods
- PMID: 26212714
- PMCID: PMC8152947
- DOI: 10.1002/14651858.MR000042.pub2
Comparison of self-administered survey questionnaire responses collected using mobile apps versus other methods
Abstract
Background: Self-administered survey questionnaires are an important data collection tool in clinical practice, public health research and epidemiology. They are ideal for achieving a wide geographic coverage of the target population, dealing with sensitive topics and are less resource-intensive than other data collection methods. These survey questionnaires can be delivered electronically, which can maximise the scalability and speed of data collection while reducing cost. In recent years, the use of apps running on consumer smart devices (i.e., smartphones and tablets) for this purpose has received considerable attention. However, variation in the mode of delivering a survey questionnaire could affect the quality of the responses collected.
Objectives: To assess the impact that smartphone and tablet apps as a delivery mode have on the quality of survey questionnaire responses compared to any other alternative delivery mode: paper, laptop computer, tablet computer (manufactured before 2007), short message service (SMS) and plastic objects.
Search methods: We searched MEDLINE, EMBASE, PsycINFO, IEEEXplore, Web of Science, CABI: CAB Abstracts, Current Contents Connect, ACM Digital, ERIC, Sociological Abstracts, Health Management Information Consortium, the Campbell Library and CENTRAL. We also searched registers of current and ongoing clinical trials such as ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform. We also searched the grey literature in OpenGrey, Mobile Active and ProQuest Dissertation & Theses. Lastly, we searched Google Scholar and the reference lists of included studies and relevant systematic reviews. We performed all searches up to 12 and 13 April 2015.
Selection criteria: We included parallel randomised controlled trials (RCTs), crossover trials and paired repeated measures studies that compared the electronic delivery of self-administered survey questionnaires via a smartphone or tablet app with any other delivery mode. We included data obtained from participants completing health-related self-administered survey questionnaire, both validated and non-validated. We also included data offered by both healthy volunteers and by those with any clinical diagnosis. We included studies that reported any of the following outcomes: data equivalence; data accuracy; data completeness; response rates; differences in the time taken to complete a survey questionnaire; differences in respondent's adherence to the original sampling protocol; and acceptability to respondents of the delivery mode. We included studies that were published in 2007 or after, as devices that became available during this time are compatible with the mobile operating system (OS) framework that focuses on apps.
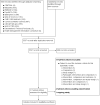
Data collection and analysis: Two review authors independently extracted data from the included studies using a standardised form created for this systematic review in REDCap. They then compared their forms to reach consensus. Through an initial systematic mapping on the included studies, we identified two settings in which survey completion took place: controlled and uncontrolled. These settings differed in terms of (i) the location where surveys were completed, (ii) the frequency and intensity of sampling protocols, and (iii) the level of control over potential confounders (e.g., type of technology, level of help offered to respondents). We conducted a narrative synthesis of the evidence because a meta-analysis was not appropriate due to high levels of clinical and methodological diversity. We reported our findings for each outcome according to the setting in which the studies were conducted.
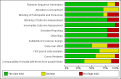
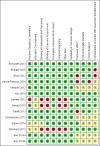
Main results: We included 14 studies (15 records) with a total of 2275 participants; although we included only 2272 participants in the final analyses as there were missing data for three participants from one included study.Regarding data equivalence, in both controlled and uncontrolled settings, the included studies found no significant differences in the mean overall scores between apps and other delivery modes, and that all correlation coefficients exceeded the recommended thresholds for data equivalence. Concerning the time taken to complete a survey questionnaire in a controlled setting, one study found that an app was faster than paper, whereas the other study did not find a significant difference between the two delivery modes. In an uncontrolled setting, one study found that an app was faster than SMS. Data completeness and adherence to sampling protocols were only reported in uncontrolled settings. Regarding the former, an app was found to result in more complete records than paper, and in significantly more data entries than an SMS-based survey questionnaire. Regarding adherence to the sampling protocol, apps may be better than paper but no different from SMS. We identified multiple definitions of acceptability to respondents, with inconclusive results: preference; ease of use; willingness to use a delivery mode; satisfaction; effectiveness of the system informativeness; perceived time taken to complete the survey questionnaire; perceived benefit of a delivery mode; perceived usefulness of a delivery mode; perceived ability to complete a survey questionnaire; maximum length of time that participants would be willing to use a delivery mode; and reactivity to the delivery mode and its successful integration into respondents' daily routine. Finally, regardless of the study setting, none of the included studies reported data accuracy or response rates.
Authors' conclusions: Our results, based on a narrative synthesis of the evidence, suggest that apps might not affect data equivalence as long as the intended clinical application of the survey questionnaire, its intended frequency of administration and the setting in which it was validated remain unchanged. There were no data on data accuracy or response rates, and findings on the time taken to complete a self-administered survey questionnaire were contradictory. Furthermore, although apps might improve data completeness, there is not enough evidence to assess their impact on adherence to sampling protocols. None of the included studies assessed how elements of user interaction design, survey questionnaire design and intervention design might influence mode effects. Those conducting research in public health and epidemiology should not assume that mode effects relevant to other delivery modes apply to apps running on consumer smart devices. Those conducting methodological research might wish to explore the issues highlighted by this systematic review.
Conflict of interest statement
JMB: none to report.
JJ: none to report.
KH: none to report.
JOD: none to report.
CPM: none to report.
JC: none to report.
Figures












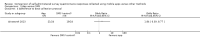


Update of
- doi: 10.1002/14651858.MR000042
References
References to studies included in this review
Ainsworth 2013 {published data only}
-
- Ainsworth J, Palmier‐Claus JE, Machin M, Barrowclough C, Dunn G, Rogers A, et al. A comparison of two delivery modalities of a mobile phone‐based assessment for serious mental illness: native smartphone application vs text‐messaging only implementations. Journal of Medical Internet Research 2013;15(4):e60. - PMC - PubMed
Brunger 2015 {published data only}
-
- Brunger L, Smith A, Re R, Wickham M, Philippides A, Watten P, et al. Validation of an iPad visual analogue rating system for assessing appetite and satiety. Appetite 2015;84:259‐63. - PubMed
Bush 2013 {published data only}
-
- Bush NE, Skopp N, Smolenski D, Crumpton R, Fairall J. Behavioral screening measures delivered with a smartphone app: psychometric properties and user preference. Journal of Nervous and Mental Disease 2013;201(11):991‐5. - PubMed
Garcia‐Palacios 2014 {published data only}
-
- Garcia‐Palacios A, Herrero R, Belmonte MA, Castilla D, Guixeres J, Molinari G, et al. Ecological momentary assessment for chronic pain in fibromyalgia using a smartphone: a randomized crossover study. European Journal of Pain 2014;18(6):862‐72. - PubMed
Khraishi 2012 {published data only}
-
- Khraishi M, Aslanov R. The use of an Apple iPad‐based© health assessment questionnaire (HAQ) application in psoriatic and rheumatoid arthritis. Dermatology and Therapy 2012;2(10):S58.
-
- Khraishi M, Aslanov R, Ogunyemi B, Gu M, Lin L. Comparing touch screen Apple iPad‐based© Health Assessment Questionnaire (HAQ) to the paper version in patients with inflammatory arthritis. Journal of Rheumatology 2012;39(8):1752.
Kim 2014 {published data only}
-
- Kim JH, Kwon SS, Shim SR, Sun HY, Ko YM, Chun DI, et al. Validation and reliability of a smartphone application for the International Prostate Symptom Score questionnaire: a randomized repeated measures crossover study. Journal of Medical Internet Research 2014;16(2):e38. [DOI: 10.2196/jmir.3042] - DOI - PMC - PubMed
Lamber 2012 {published data only}
-
- Lamber P, Mitterer M, Napolitano L, Ricci F, Zini F. Surveying patients with smart devices. 25th International Symposium on Computer‐Based Medical Systems. 2012 Jun 20‐22:1‐4.
Newell 2015 {published data only}
Salaffi 2013 {published data only}
-
- Salaffi F, Gasparini S, Ciapetti A, Gutierrez M, Grassi W. Usability of an innovative and interactive electronic system for collection of patient‐reported data in axial spondyloarthritis: comparison with the traditional paper‐administered format. Rheumatology 2013;52(11):2062‐70. - PubMed
Schemmann 2013 {published data only}
-
- Schemmann D, Rudolph J, Haas H, Müller‐Stromberg J. Validation and patient acceptance of a touch tablet version of the iHOT‐12 questionnaire. Arthroscopy 2013;29(12 (Suppl)):e188.
Sigaud 2014 {published data only}
-
- Sigaud M, Horvais V, Chamouard V, Guillet B, Lambert T, Borel‐Derlon A, et al. Comparison of paper diary and B‐CoNect (telemetric smartphone application) at home treatment monitoring of severe hemophilia A patients. Haemophilia 2014;20(Suppl. 3):180.
Stomberg 2012 {published data only}
Sun 2013a {published data only}
-
- Sun T, West N, Ansermino M, Montgomery C, Lauder G, Baeyer C. PANDA: Evaluation of a smartphone‐based perioperative pain assessment tool. Journal of Investigative Medicine 2013;61(1):115.
-
- Sun T, West N, Ansermino M, Montgomery CJ, Myers D, Baeyer CL, et al. PANDA: Evaluation of a smartphone‐based peri‐operative pain assessment tool. Canadian Journal of Anesthesia 2013;60:S25.
Sun 2013b {published data only}
-
- Sun T, West N, Ansermino M, Montgomery C, Lauder G, Baeyer C. PANDA: Evaluation of a smartphone‐based perioperative pain assessment tool. Journal of Investigative Medicine 2013;61(1):115.
-
- Sun T, West N, Ansermino M, Montgomery CJ, Myers D, Baeyer CL, et al. PANDA: Evaluation of a smartphone‐based peri‐operative pain assessment tool. Canadian Journal of Anesthesia 2013;60:S25.
References to studies excluded from this review
Abernethy 2008 {published data only}
-
- Abernethy AP, Herndon JE 2nd, Wheeler JL, Patwardhan M, Shaw H, Lyerly HK, et al. Improving health care efficiency and quality using tablet personal computers to collect research‐quality, patient‐reported data. Health Services Research 2008;43(6):1975‐91. [DOI: 10.1111/j.1475-6773.2008.00887.x] - DOI - PMC - PubMed
Ahmad 2012 {published data only}
Aktas 2014 {published data only}
-
- Aktas A, Hullihen B, Shrotriya S, Thomas S, Walsh D, Estfan B. Connected health: a pilot study of cancer symptom and quality of life assessment with a tablet computer. Palliative Medicine 2014;28:755‐6. - PubMed
Alhajji 2009 {published data only}
-
- Alhajji M, Jeffrey A, Datta A. Tablet PC to evaluate respiratory patient preference and satisfaction using the 18‐element Consultation Specific Questionnaire. Thorax 2009;64(Suppl IV):A90. [DOI: 10.1136/thx.2009.127134i] - DOI
Allena 2012 {published data only}
Alsip 2014 {published data only}
-
- Alsip C, Rich A. Using iPads to improve patient care. Radiology Management 2014;36(2):20‐1. - PubMed
Bakshi 2013a {published data only}
-
- Bakshi N, Stinson J, Lukombo I, Ross D, Mittal N, Vinod Joshi S, et al. Development, establishment of the psychometric properties and usability testing of a novel multi‐dimensional web based diary for children with sickle cell disease. Blood 2013;122:21.
Bakshi 2013b {published data only}
-
- Bakshi N, Stinson J, Rice K, Ross D, Krishnamurti L. Preliminary validation of a multi‐dimensional electronic pain diary for children with sickle cell disease. Pediatric Blood and Cancer 2013;60:S31.
Barentsz 2014 {published data only}
Bartlett 2013a {published data only}
-
- Bartlett SJ, Orbai AM, Duncan T, Bingham III CO. How well do generic patient reported outcomes measurement information system instruments capture health status in rheumatoid arthritis?. Arthritis & Rheumatism 2013;65(10 (Supplement)):S972.
Bartlett 2013b {published data only}
-
- Bartlett SJ, Orbai AM, Duncan T, Leon E, Jones M, Bingham III CO. Preliminary data supporting the feasibility and construct validity of promis fatigue instrument in an academic rheumatoid arthritis clinic. Annals of the Rheumatic Diseases 2013;72(Suppl 3):585.
Beasley 2008 {published data only}
Bellamy 2009a {published data only}
-
- Bellamy N, Wilson C, Hendrikz J, Patel B, Dennison S. Electronic data capture (EDC) using cellular technology: implications for clinical trials and practice, and preliminary experience with the m‐Womac Index in hip and knee OA patients. Inflammopharmacology 2009;17(2):93‐9. [DOI: 10.1007/s10787-008-8045-4] - DOI - PubMed
Bellamy 2009b {published data only}
-
- Bellamy N, Wilson C, Hendrikz J, Patel B, Dennison S. Validation study of electronic data capture (EDC) using the WOMAC® NRS 3.1 Index (m‐WOMAC®): preliminary interim analysis. Internal Medicine Journal 2009;39(Suppl 2):A67.
Bellamy 2011a {published data only}
Bellamy 2011b {published data only}
-
- Bellamy N, Hendrikz J, Wilson C. Comparison of transformed visual analogue and native numerical rating scaled patient responses to the WOMAC® Index. Internal Medicine Journal 2011;41(Suppl 1):23.
Ben‐Zeev 2012 {published data only}
Bernabe‐Ortiz 2008 {published data only}
Bernhardt 2009 {published data only}
Berry 2014 {published data only}
Bethoux 2014 {published data only}
-
- Bethoux F, Rudick R, Miller D, Rao S, Lee JC, Stough D, et al. The Multiple Sclerosis Performance Test: an innovative approach to measuring MS‐related manual dexterity impairment. Neurology 2014;82(10 (Supplement)):P3.132.
Bexelius 2010 {published data only}
Blaivas 2013a {published data only}
-
- Blaivas JG, Weinberger JM, Weiss JP, Kashan M. Validation of an electronic bladder diary application. Journal of Urology 2013;189(4S):e804‐5.
Blaivas 2013b {published data only}
-
- Blaivas JG, Weinberger JM, Weiss JP, Kashan M. Validation of WESHARETM: a new bladder diary application. Neurourology and Urodynamics 2013;32(2):119.
Blum 2014 {published data only}
-
- Blum D, Koeberle D, Omlin A, Walker J, Moos R, Mingrone W, et al. Feasibility and acceptance of electronic monitoring of symptoms and syndromes using a handheld computer in patients with advanced cancer in daily oncology practice. Support Care Cancer 2014;22(9):2425‐34. [DOI: 10.1007/s00520-014-2201-8] - DOI - PubMed
Bockenek 2014 {published data only}
-
- Bockenek JA, Brooks BR, Sanjak MS, Lucas NM, Smith NP, Nichols MS, et al. New disease management tools – Cerner electronic medical record deployment of Amyotrophic Lateral Sclerosis Functional Rating Scale‐Revised (ALSFRS‐R) validated with mobile smartphone (iPhone/Android) application (ALSFRSR‐Lite). Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 2014;15(Suppl 1):121‐2. [DOI: 10.3109/21678421.2014.960178/122] - DOI
Bokhour 2013 {published data only}
-
- Bokhour BG, Solomon J, Laws MB, Gifford AL, Goetz MB. The impact of an HIV adherence informatics intervention on patient‐provider communication about ART adherence. Poster presentation at the Society of General Internal Medicine Annual Meeting. Denver, CO, 2013 Apr 25‐27.
Bond 2013 {published data only}
-
- Bond S, Devitt B, Lane H, McLachlan SA, Philip J. Can older patients use an electronic tablet to complete a cancer specific geriatric assessment? Results of a pilot study. Asia‐Pacific Journal of Clinical Oncology 2013;9(Suppl 3):150.
Boushey 2009 {published data only}
Bradbury 2012 {published data only}
-
- Bradbury A, Bate G, Thomas E, King T, Wright D. Varicose Veins (VV) Symptoms Questionnaire: a simple, validated measure of VV symptoms that can be administered daily using a Personal Digital Assistant (PDA). Journal of Vascular Surgery 2012;55(6):64‐5.
Braun 2008 {published data only}
-
- Braun MD, Elliot N, Pantel A. Rangeland health data collection and analysis improved with mobile GIS. 2008. ArcNews Online (accessed 07 August 2014). [http://www.esri.com/news/arcnews/spring08articles/rangeland‐health.html]
Burke 2009 {published data only}
Buskirk 2014 {published data only}
-
- Buskirk TD, Andrus CH. Making mobile browser surveys smarter: results from a randomized experiment comparing online surveys completed via computer or smartphone. Field Methods 2014;26(4):322‐42.
Carter 2013a {published data only}
Carter 2013b {published data only}
Christie 2013 {published data only}
-
- Christie A, Dagfinrud H, Dale Ø, Schulz T, Hagen KB. Why use pen and paper in data collection when you can use a mobile phone? ‐ comparison of the two methods. Annals of the Rheumatic Diseases 2013;72(Suppl 3):1053.
Clionsky 2014 {published data only}
Cook 2007 {published data only}
-
- Cook IA, Balasubramani GK, Eng H, Friedman E, Young EA, Martin J, et al. Electronic source materials in clinical research: acceptability and validity of symptom self‐rating in major depressive disorder. Journal of Psychiatric Research 2007;41(9):737‐43. [DOI: 10.1016/j.jpsychires.2006.07.015] - DOI - PubMed
Croff 2012 {published data only}
-
- Croff JM. Feasibility of using iPads for data collection at college parties. Alcoholism: Clinical and Experimental Research 2012;36:66A.
Cudlip 2014 {published data only}
-
- Cudlip F, Swatzell V, Alexandrov DA, Freier MR, Rowek T, Wojner AJ, et al. Feasibility of an electronic point‐of‐discharge tablet for collection of perception of stroke care quality data. Stroke 2014;45:AN55.
Cunningham 2013 {published data only}
Dale 2007 {published data only}
de Bruijne 2013 {published data only}
-
- Bruijne M, Wijnant A. Comparing survey results obtained via mobile devices and computers: an experiment with a mobile web survey on a heterogeneous group of mobile devices versus a computer‐assisted web survey. Social Science Computer Review 2013;31(4):482‐504. [DOI: 10.1177/0894439313483976] - DOI
DeMaria 2012 {published data only}
-
- DeMaria AN. Self quantification of health and fitness. Journal of the American College of Cardiology 2012;60(16):1574‐5. - PubMed
Denny 2008 {published data only}
Depp 2012 {published data only}
Desai 2012 {published data only}
-
- Desai S, Witkiewitz K, Bowen S, Larimer M. The moderating effect of behavioral monitoring on the association between urgency and drinking outcomes among college students. Alcoholism: Clinical and Experimental Research 2012;36:243A.
Dewit 2012 {published data only}
-
- Dewit MA. Evaluating the impact of an iPad application on the efficiency and accuracy of data collection in clinical sessions of Acceptance and Commitment Therapy. Carbondale, IL: Southern Illinois University Carbondale, 2012.
Duncan 2012 {published data only}
-
- Duncan MJ, Vandelanotte C, Rosenkranz RR, Caperchione CM, Ding H, Ellison M, et al. Effectiveness of a website and mobile phone based physical activity and nutrition intervention for middle‐aged males: trial protocol and baseline finding of the ManUp Study. BMC Public Health 2012;12:656. - PMC - PubMed
Dupont 2009 {published data only}
-
- Dupont A, Wheeler J, Herndon JE 2nd, Coan A, Zafar SY, Hood L, et al. Use of tablet personal computers for sensitive patient‐reported information. Journal of Supportive Oncology 2009;7(3):91‐7. - PubMed
Dy 2012 {published data only}
-
- Dy CJ, Schmicker T, Tran Q, Chadwick B, Daluiski A. The use of a tablet computer to complete the DASH questionnaire. Journal of Hand Surgery 2012;37(12):2589‐94. - PubMed
Edwards 2008 {unpublished data only}
-
- Edwards JF. The Pyschometric Equivalency of Scores from a Web‐Based Questionnaire Administered via Cellphone versus Desktop Computer. Mississippi State University, 2008.
Escandon 2008 {published data only}
Eskenazi 2014 {published data only}
Fanning 2014 {published and unpublished data}
Farach 2013 {published data only}
-
- Farach N, Galindo H, Tinajeros F, Guardado M. Use of tablets for data collection among female sex workers: lessons learned from a behavioural surveillance study in Honduras, 2012. Sexually Transmitted Infections 2013;89:A248. [DOI: 10.1136/sextrans-2013-051184.0771] - DOI
Faurholt‐Jepsen 2013 {published data only}
-
- Faurholt‐Jepsen M, Vinberg M, Christenses EM, Frost M, Bardram J, Kessing LV. Daily electronic self‐monitoring of subjective and objective symptoms in bipolar disorder ‐ the MONARCA trial protocol (MONitoring, treATment and pRediCtion of bipolAr disorder episodes): a randomised controlled single‐blind trial. BMJ Open 2013;3(7):e003353. [DOI: 10.1136/bmjopen-2013-003353] - DOI - PMC - PubMed
Fritz 2012 {published data only}
-
- Fritz F, Balhorn S, Riek M, Breil B, Dugas M. Qualitative and quantitative evaluation of EHR‐integrated mobile patient questionnaires regarding usability and cost‐efficiency. International Journal of Medical Informatics 2012;81(5):303‐13. - PubMed
Galliber 2008 {published data only}
Garcia 2010 {published data only}
-
- Garcia Vega OA, Buendía Rodriguez JA. Concordance among three self‐reported measures of medication adherence and count of tablets records in Colombian hypertensive patients. Value in Health 2010;13(3):A167.
Gibbons 2011 {published data only}
Giesinger 2013 {published data only}
Glaser 2013 {published data only}
Goldstein 2011 {published data only}
Gupta 2013 {published data only}
-
- Gupta A, Thapar J, Singh A, Singh P, Srinivasan V, Vardhan V. Simplifying and improving mobile based data collection. Proceedings of the Sixth International Conference on Information and Communications Technologies and Development. Cape Town, South Africa: ACM, 2013 Dec 07‐10:45‐8.
Gurland 2010a {published data only}
-
- Gurland B, Ferreira PCA, Sobol T, Kiran RP. Using technology to facilitate data capture and integration of patient reported outcomes (PRO) into colorectal surgical practice. Colorectal Disease 2010;12(Suppl 1):45.
Gurland 2010b {published data only}
-
- Gurland B, Alves‐Ferreira PC, Sobol T, Kiran RP. Using technology to improve data capture and integration of patient‐reported outcomes into clinical care: pilot results in a busy colorectal unit. Diseases of the Colon & Rectum 2010;53:1168‐75. - PubMed
Hallum‐Montes 2013 {published data only}
-
- Hallum‐Montes RM, Opdyke KM, Ruggeri C, Aliaga M, Bal D, Hurlbert M, et al. Implementation of electronic client‐level data collection via tablet technology among organizations supported by the Avon breast health outreach program. Cancer Research 2013;73:P6‐08‐15.
Harralson 2013 {published data only}
-
- Harralson T, Toche‐Manley L, Dietzen L, Grissom G, O'Hea E, Boudreaux E. Development and implementation of an automated distress management system for cancer patients. American Psychosocial Oncology Society 10th Annual Conference. 2013 Feb 13‐15.
Harris 2013 {published data only}
-
- Harris MA, Duke DC, Raymond JK, Harris JB, Shimomaeda L, Shimomaeda K. Self‐administered diabetes self‐management profile (SA‐DSMP): reliability, validity, and utility. Diabetes 2013;62:A636.
Hashemian 2012 {published data only}
-
- Hashemian M, Knowles D, Calver J, Qian W, Bullock MC, Bell S, et al. iEpi: An end to end solution for collecting, conditioning and utilizing epidemiologically relevant data. Proceedings of the 2nd ACM International Workshop on Pervasive Wireless Healthcare. Hilton Head, South Carolina, USA: ACM, 2012 Jun 11‐14:3‐8.
Haver 2011 {published data only}
-
- Haver AE, Marcotulio N, Elliott JP. Utilization of iPads to facilitate data collection during pediatric screening events. Pharmacotherapy 2011;31(10):418e‐9e.
Heiberg 2007 {published data only}
-
- Heiberg T, Kvien TK, Dale Ø, Mowinckel P, Aanerud GJ, Songe‐Møller AB, et al. Daily health status registration (patient diary) in patients with rheumatoid arthritis: a comparison between personal digital assistant and paper‐pencil format. Arthritis and Rheumatism 2007;57(3):454‐60. - PubMed
Hollen 2013 {published data only}
-
- Hollen PJ, Gralla RJ, Stewart JA, Meharchand JM, Wierzbicki R, Leighl N. Can a computerized format replace a paper form in PRO and HRQL evaluation? Psychometric testing of the computer‐assisted LCSS instrument (eLCSS‐QL). Supportive Care in Cancer 2013;21(1):165‐72. - PubMed
Huang 2010 {published data only}
-
- Huang F, Zhu ZH, Wang WZ, Zhang JX, Ji Y, Zhang K, et al. Psychometric equivalence between mobile phone based and paper‐and‐pencil tests: a case with children's revised impact of event scale. Chinese Journal of Clinical Psychology 2010;18(1):31‐3.
Huang 2012 {published data only}
-
- Zirong H, Junqing W, Coffey PS, Kilbourne‐Brook M, Yufeng Z, Wang C, et al. Performance of the woman's condom among couples in Shanghai, China. European Journal of Contraception and Reproductive Health Care 2012;17(3):212‐8. - PubMed
Huguet 2014 {published data only}
Hundeshagen 2013 {published data only}
-
- Hundeshagen G, Weissman O, Farber N, Winkler E, Haik J. Approaches to clinical research: alternative use of the Google Docs survey function for mobile data collection using physicians' smartphones. Plastic & Reconstructive Surgery 2013;131(1):135e‐6e. - PubMed
Hutchesson 2015 {published data only}
-
- Hutchesson MJ, Rollo ME, Callister R, Collins CE. Self‐monitoring of dietary intake by young women: online food records completed on computer or smartphone are as accurate as paper‐based food records but more acceptable. Journal of the Academy of Nutrition and Dietetics 2015;115(1):87‐94. - PubMed
Isara 2013 {published data only}
-
- Isara AR, Onyeagwara NC, Lawin H, Irabor I, Igwenyi C, Kabamba L. Survey of airflow obstruction in two African countries: paper questionnaire versus mobile phone technology. African Journal of Respiratory Medicine 2013;8(2):13‐6.
Jacob 2012 {published data only}
Jaspan 2007 {published data only}
-
- Jaspan HB, Flisher AJ, Myer L, Mathews C, Seebregts C, Berwick JR, et al. Brief report: methods for collecting sexual behaviour information from South African adolescents ‐ a comparison of paper versus personal digital assistant questionnaires. Journal of Adolescence 2007;30(2):353‐9. - PubMed
Johnson 2014 {published data only}
Juniper 2009 {published data only}
-
- Juniper EF, Langlands JM, Juniper BA. Patients may respond differently to paper and electronic versions of the same questionnaires. Respiratory Medicine 2009;103(6):932‐4. - PubMed
Junker 2008 {published data only}
-
- Junker U, Freynhagen R, Längler K, Gockel U, Schmidt U, Tölle TR, et al. Paper versus electronic rating scales for pain assessment: a prospective, randomised, cross‐over validation study with 200 chronic pain patients. Current Medical Research and Opinion 2008;24(6):1797‐806. - PubMed
Kajander 2007 {published data only}
-
- Kajander K, Lätti M, Hatakka K, Korpela R. An electronic diary versus a paper diary in measuring gastrointestinal symptoms. Digestive and Liver Disease 2007;39(3):288‐9. - PubMed
Kauer 2012 {published data only}
Kaufman 2013 {published data only}
-
- Kaufman ZA, Hershow R, DeCelles J, Bhauti K, Dringus S, Delany‐Moretlwe S, et al. Acceptability of data collection on mobile phones using ODK software for self‐administered sexual behaviour questionnaires. Sexually Transmitted Infections 2013;89(Suppl 1):A249.
Kelly 2014 {published data only}
Khair 2014a {published data only}
-
- Khair K, Barrie A, Hubert N, Holland M. Pedhal goes electronic ‐ results of a single centre pilot study. Haemophilia 2014;20(Suppl 2):33.
Khair 2014b {published data only}
-
- Khair K, Hubert N, Barri A, Spires J, Griffioen A, Holland M. IPad‐based PedHAL app is intuitive and acceptable to boys with hemophilia. Haemophilia 2014;20(Suppl 3):84.
Khor 2014a {published data only}
-
- Khor AS, Gray KM, Reid SC, Melvin GA. Feasibility and validity of ecological momentary assessment in adolescents with high‐functioning autism and Asperger's disorder. Journal of Adolescence 2014;37(1):37‐46. - PubMed
Khor 2014b {published data only}
-
- Khor AS, Melvin GA, Reid SC, Gray KM. Coping, daily hassles and behavior and emotional problems in adolescents with high‐functioning autism/Asperger's disorder. Journal of Autism and Developmental Disorders 2014;44(3):593‐608. - PubMed
Khraishi 2013 {published data only}
-
- Khraishi M, Aslanov R, Fudge K. The validation of a new simple disease activity tool in Systemic Lupus Erythematosus (SLE): the Lupus Activity Scoring Tool (LAST) as compared to the SLEDAI SELENA modification. Lupus 2013;22(1):70‐1.
Kimel 2010 {published data only}
-
- Kimel M, McCormak J, Chen WH, Brunt K, Runken MC. A comparative trial of paper‐and‐pencil versus electronic administration of the Patient Perception of Migraine Questionnaire‐Revised (PPMQ‐R). 52nd Annual Scientific Meeting of the American Headache Society. Los Angeles, CA, 2010 Jun 24‐27.
King 2013 {published data only}
Kirwan 2012 {published data only}
Kochan 2007 {published data only}
-
- Kochan B, Bellemans T, Janssens D, Wets G, Timmermans H. Paper‐and‐pencil versus personal digital assistant enabled surveys: a comparison. Transportation Systems: Engineering & Management. 12th Conference of the Hong‐Kong Society for Transportation Studies. Hong Kong University Science & Technology, 2007 Dec 08‐10.
Krogh 2013 {published and unpublished data}
-
- Krogh AB, Larsson B, Linde M. Comparing electronic and paper diary recordings of headache among adolescents in the general population. Cephalalgia 2013;33(8):142‐3. - PubMed
Kuntsche 2013 {published data only}
Kuntsche 2014 {published data only}
-
- Kuntsche E, Labhart F. The future is now‐‐using personal cellphones to gather data on substance use and related factors. Addiction 2014;109(7):1052‐3. - PubMed
Lam 2010 {published data only}
-
- Lam J, Barr RG, Catherine N, Tsui H, Hahnhaussen CL, Pauwels J, et al. Electronic and paper diary recording of infant and caregiver behaviors. Journal of Developmental and Behavioral Pediatrics 2010;31(9):685‐93. - PubMed
Lange 2014 {published data only}
-
- Lange S, Süß HM. Measuring slips and lapses when they occur ‐ ambulatory assessment in application to cognitive failures. Consciousness and Cognition 2014;24:1‐11. - PubMed
Lee 2010 {published data only}
-
- Lee IJ, Huang S‐Y, Tsou M‐Y, Chan K‐H, Chang K‐Y. Decision analysis for a data collection system of patient‐controlled analgesia with a multi‐attribute utility model. Journal of the Chinese Medical Association 2010;73(10):533‐9. - PubMed
Lee 2014 {published data only}
-
- Lee H, Ahn H, Choi S, Choi W. The SAMS: Smartphone Addiction Management System and verification. Journal of Medical Systems 2014;38(1):1. - PubMed
Levine 2012 {published data only}
Lundy 2013 {published data only}
-
- Lundy JJ. Implementing New COA Instruments on Alternative Data Collection Modes: The Electronic Implementation Assessment. Value in Health 2013;16(3):A39.
Malotte 2011 {published data only}
-
- Malotte C, Cutting A, Huettner S, Matson P, Ellen J. Feasibility of using cell phones for daily data collection within adolescent cohort studies. Sexually Transmitted Infections 2011;87:A260‐1.
Mangera 2014 {published data only}
-
- Mangera A, Marzo A, Heron N, Fernando D, Hameed K, Soliman A‐HA, et al. Development of two electronic bladder diaries: a patient and healthcare professionals pilot study. Neurourology and Urodynamics 2014;33(7):1101–9. - PubMed
Marceau 2007 {published data only}
-
- Marceau LD, Link C, Jamison RN, Carolan S. Electronic diaries as a tool to improve pain management: is there any evidence?. Pain Medicine 2007;8(Suppl 3):S101‐9. - PubMed
Marceau 2010 {published data only}
Martin 2012 {published data only}
-
- Martin P, Brown C, Cuffe S, Pringle D, Mahler M, Villeneuve J, et al. Use of iPad technology to determine cancer patient‐reported preferences for and understanding of pharmacogenetic testing (PGT). Journal of Clinical Oncology 2012;Suppl 34:Abstract 319.
Matthew 2007a {published data only}
Matthew 2007b {published data only}
Mavletova 2013 {published data only}
Mays 2010 {published data only}
-
- Mays D, Cremeens J, Usdan S, Martin RJ, Arriola KJ, Bernhardt JM. The feasibility of assessing alcohol use among college students using wireless mobile devices: Implications for health education and behavioural research. Health Education Journal 2010;69(3):311‐20.
McCaw 2010 {published data only}
McIntosh 2013 {published data only}
-
- McIntosh LD, Black L, Morley J, Long S, Carter P, Jones E, et al. Assessing the feasibility of electronic data collection for men with prostate cancer. Journal of Urology 2013;189(4S):e185‐6.
Michalak 2009 {published and unpublished data}
-
- Michalak EE, Kreindler DM, Murray G, Suto M, Johnson S, Amari E, et al. Mood monitoring in bipolar disorder: a hand‐held computer intervention. Bipolar Disorders 2009;11(Suppl 1):63.
Miller 2013 {published data only}
-
- Miller DP, Denizard‐Thompson NM, Wofford JL, Babcock D, Weaver KE, Case LD, et al. iPad‐based patient education and data collection for colorectal cancer screening. Journal of General Internal Medicine 2013;28:S245‐6.
Mulvaney 2012 {published data only}
Nishiguchi 2014 {published data only}
-
- Nishiguchi S, Ito H, Yamada M, Yoshitomi H, Furu M, Ito T, et al. Self‐assessment tool of disease activity of rheumatoid arthritis by using a smartphone application. Telemedicine Journal and e‐Health 2014;20(3):235‐40. - PubMed
Oliver 2013 {published data only}
-
- Oliver E, Baños RM, Cebolla A, Lurbe E, Alvarez‐Pitti J, Botella C. An electronic system (PDA) to record dietary and physical activity in obese adolescents; data about efficiency and feasibility [Un sistema electrónico (PDA) para el registro de ingesta y actividad física en adolescentes obesos; datos sobre eficiencia y viabilidad]. Nutrición Hospitalaria 2013;28(6):1860‐6. [DOI: 10.3305/nh.2013.28.6.6784] - DOI - PubMed
Pakhare 2013 {published data only}
-
- Pakhare AP, Bali S, Kalra G. Use of mobile phones as research instrument for data collection. Indian Journal of Community Health 2013;25(2):95‐8.
Patel 2012 {published data only}
Patnaik 2009 {published data only}
-
- Patnaik S, Brunskill E, Thies W. Evaluating the accuracy of data collection on mobile phones: a study of forms, SMS, and voice. International Conference on Information and Communication Technologies and Development. 2009 Apr 17‐19:74‐84.
Pau 2013 {published data only}
-
- Pau D, Nguyen L, Pibre S, Gokou S, Paget J. Results of a study using a tablet PC to collect PROS in elderly population. Value in Health 2013;16(7):A604.
Pfaeffli 2013 {published data only}
Phillips 2014 {published data only}
-
- Phillips KA, Epstein DH, Jobes ML, Preston KL. Smartphone‐reported stress and drug events and day‐end perceived stress, hassles, and mood in methadone‐maintained individuals. Journal of General Internal Medicine 2014;29:S209‐10.
Polak 2014 {published data only}
-
- Polak E, Apfel A, Privitera M, Buse D, Haut S. Daily diaries in epilepsy research: does electronic format improve adherence?. Epilepsy Currents 2014;14:180.
Quadri 2012 {published data only}
-
- Quadri N, Langel K, Muehlhausen W, O'Donohoe P, Wild D. Exploring patient perceptions of, and preferences for, pain response scales. Value in Health 2012;15:A482.
Rao 2014 {published data only}
-
- Rao S, Alberts J, Miller D, Bethoux F, Lee JC, Stough D, et al. Processing Speed Test (PST): A self‐administered iPad®‐based tool for assessing MS‐related cognitive dysfunction. Neurology 2014;82(10 (Suppl)):S33.001.
Raptis 2011 {unpublished data only}
-
- Raptis DA, Rolf G. Desktop Versus Mobile Data Collection in Clinical Trial. ClinicalTrials.gov 2011. [NCT01473238]
Richter 2008 {published data only}
Ring 2008 {published data only}
-
- Ring AE, Cheong KA, Watkins CL, Meddis D, Cella D, Harper PG. A randomized study of electronic diary versus paper and pencil collection of patient‐reported outcomes in patients with non‐small cell lung cancer. Patient 2008;1(2):105‐13. - PubMed
Roth 2014 {published data only}
Runyan 2013 {published data only}
Russman 2014 {published data only}
-
- Russman A, Hirsch J, Schindler D, Burke D, Linder S, Alberts J. Validation of a self‐administered iPad®‐ and iPod®‐based tool for assessing information processing. Neurology 2014;82(10 (Suppl)):P5.302.
Sage 2012 {published and unpublished data}
-
- Sage JM, Ali A, Farrell J, Huggins JL, Covert K, Eskra D, et al. Moving into the electronic age: validation of rheumatology self‐assessment questionnaires on tablet computers. Arthritis & Rheumatism 2012; Vol. 64, issue Suppl:S1102.
Sander 2012 {published data only}
-
- Sander P, Chung S, Ellen J, Matson P. Missing data in a mobile phone daily diary study of adolescents. American Journal of Epidemiology 2012;175(11 Suppl):S137.
Scheers 2012 {published data only}
-
- Scheers T, Philippaerts R, Lefevre J. Assessment of physical activity and inactivity in multiple domains of daily life: a comparison between a computerized questionnaire and the SenseWear Armband complemented with an electronic diary. International Journal of Behavioral Nutrition and Physical Activity 2012;9:71. - PMC - PubMed
Schlechtweg 2013 {published data only}
Seebregts 2009 {published data only}
-
- Seebregts CJ, Zwarenstein M, Mathews C, Fairall L, Flisher AJ, Seebregts C, et al. Handheld computers for survey and trial data collection in resource‐poor settings: development and evaluation of PDACT, a Palm Pilot interviewing system. International Journal of Medical Informatics 2009;78(11):721‐31. - PubMed
Shafran 2009 {published data only}
-
- Shafran I, Burgunder P, Shamosh B. Mobile and web‐based application for IBD tracking. Inflammatory Bowel Diseases 2009;15:S40.
Shapiro 2011 {published data only}
-
- Shapiro S, Stuckey M, Sabourin K, Munoz C, Petrella RJ. Smartphone technology versus paper‐based logs for type II diabetes prevention: psychological and behavioral outcomes. Canadian Journal of Cardiology 2011;27(5 Suppl):S173‐4.
Shay 2009 {published data only}
Short 2013 {published data only}
-
- Short J, Johnson R, Barr L, Yeh WC, Harvey J, Mathen V. iPad based applications have the potential to revolutionise cancer data collection. European Journal of Surgical Oncology 2013;39(11):S81.
Smith 2011 {published data only}
-
- Smith PH, Homish GG, Barrick C, Grier NL. Using touch‐screen technology to assess smoking in a low‐income primary care clinic: a pilot study. Substance Use & Misuse 2011;46(14):1750‐4. - PubMed
Smith 2014 {published data only}
Spark 2015 {published data only}
-
- Spark S, Lewis D, Vaisey A, Smyth E, Wood A, Temple‐Smith M, et al. Using computer‐assisted survey instruments instead of paper and pencil increased completeness of self‐administered sexual behavior questionnaires. Journal of Clinical Epidemiology 2015;68(1):94‐101. - PubMed
Sternfeld 2012 {published data only}
-
- Sternfeld B, Jiang SF, Picchi T, Chasan‐Taber L, Ainsworth B, Quesenberry CP Jr. Evaluation of a cell phone‐based physical activity diary. Medicine and Science in Sports and Exercise 2012;44(3):487‐95. - PubMed
Stukenborg 2013 {published data only}
-
- Stukenborg G, Blackhall L, Harrison J, Read P. Palliative care cancer patient reported outcomes assessment using tablets. Supportive Care in Cancer 2013;21(Suppl 1):S118‐9.
Swartz 2007 {published data only}
-
- Swartz RJ, Moor C, Cook KF, Fouladi RT, Basen‐Engquist K, Eng C, et al. Mode effects in the center for epidemiologic studies depression (CES‐D) scale: personal digital assistant vs. paper and pencil administration. Quality of Life Research 2007;16(5):803‐13. - PubMed
Tegang 2009 {published data only}
-
- Tegang SP, Emukule G, Wambugu S, Kabore I, Mwarogo P. A comparison of paper‐based questionnaires with PDA for behavioral surveys in Africa: findings from a behavioral monitoring survey in Kenya. Journal of Health Informatics in Developing Countries 2009;3(1):22‐5.
Temple 2014 {unpublished data only}
-
- Temple L, Patil S. Feasibility and Psychometric Properties of Paper vs. Web vs. Automated Telephone Administration of Patient Reported Outcome Surveys. ClinicalTrials.gov 2014. [NCT01458509]
Tolley 2013 {published data only}
-
- Tolley C, Lalonde J, Rofail D, Gater A. "It was easier than dealing with a pen and paper...": exploring the usability of electronic devices for completion of Clinical Outcome Assessments (COAs) in schizophrenia. European Archives of Psychiatry and Clinical Neuroscience 2013;263(Suppl 1):S87.
Trapl 2007 {published data only}
-
- Trapl ES. Understanding adolescent survey responses: impact of mode and other characteristics on data outcomes and quality. Dissertation Abstracts International: Section B: The Sciences and Engineering 2007; Vol. 68:2303.
Trapl 2013 {published data only}
Tully 2014 {published data only}
-
- Tully LM, Leon E, Motoru S, Wahba K, Smith P, Singh K, et al. Using a novel mobile health application to monitor symptoms and functioning in an early psychosis program: preliminary data on feasibility and acceptability. Biological Psychiatry 2014;75:387S.
Tyser 2015 {published data only}
-
- Tyser AR, Beckmann J, Weng C, O'Farrell A, Hung M. A randomized trial of the disabilities of the arm, shoulder, and hand administration: tablet computer versus paper and pencil. Journal of Hand Surgery ‐ American Volume 2015;40(3):554‐9. - PubMed
Unver 2009 {published data only}
-
- Unver YB, Yavuz GA, Sinclair SH. Interactive, computer‐based, self‐reported, visual function questionnaire: the PalmPilot‐VFQ. Eye 2009;23(7):1572‐81. - PubMed
van Duinen 2008 {published data only}
-
- Duinen M, Rickelt J, Griez E. Validation of the electronic Visual Analogue Scale of Anxiety. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 2008;32(4):1045‐7. - PubMed
van Heerden 2014 {published data only}
-
- Heerden AC, Norris SA, Tollman SM, Stein AD, Richter LM. Field lessons from the delivery of questionnaires to young adults using mobile phones. Social Science Computer Review 2014;32:105‐12.
Vargas 2010 {published data only}
Viana 2014 {published data only}
-
- Viana JS, Pombo N, Araújo P, Dias da Costa M. Evaluation of a smartphone application connected to a web based system for remote monitoring of post‐operative pain in ambulatory surgery: a randomised controlled trial. European Journal of Anaesthesiology 2014;31:225‐6.
Vinney 2012 {published and unpublished data}
Walther 2011 {published data only}
Wells 2014 {published data only}
-
- Wells T, Bailey JT, Link MW. Comparison of smartphone and online computer survey administration. Social Science Computer Review 2014;32(2):238‐55. [DOI: 10.1177/0894439313505829] - DOI
Wharton 2014 {published data only}
-
- Wharton CM, Johnston CS, Cunningham BK, Sterner D. Dietary self‐monitoring, but not dietary quality, improves with use of smartphone app technology in an 8‐week weight loss trial. Journal of Nutrition Education and Behavior 2014;46(5):440‐4. - PubMed
Wilcox 2012 {published data only}
-
- Wilcox AB, Gallagher KD, Boden‐Albala B, Bakken SR. Research data collection methods ‐ from paper to tablet computers. Medical Care 2012;50(Suppl):S68‐S73. - PubMed
Wilson 2013a {published data only}
-
- Wilson D, Wilson G, Patel P. A novel validated electronic patient data acquisition tool standardizes patient reported outcomes (PRO) data acquisition across multi‐center environmental exposure chamber and field studies. Journal of Allergy and Clinical Immunology 2013;131(2 Suppl):AB226.
Wilson 2013b {published data only}
-
- Wilson D, Nandkeshore H, Patel P. Development and validation of an electronic patient data acquisition tablet for allergy symptom collection in an environmental exposure chamber and at‐home. Allergy 2013;68(Suppl 97):466‐7.
Witt 2015 {published data only}
-
- Witt J, Brown A, Kaler P, Pannell C, Murtagh FEM. The future of data collection in palliative care practice. BMJ Supportive & Palliative Care 2015;5(1):116. [DOI: 10.1136/bmjspcare-2014-000838.36] - DOI
Wofford 2014 {published data only}
-
- Wofford JL, Campos CL, Stevens SR, Jones RE. Real‐time patient survey data during real‐time clinics: Implementing technology‐enhanced rapid‐cycle quality improvement. Journal of General Internal Medicine 2014;29:S493‐4.
Wood 2011 {published data only}
Woods 2009 {published data only}
Wundes 2011 {published data only}
-
- Wundes A, Amtmann D, Johnson K, Salem R, Yang DS, Schulz L, et al. Collecting health‐related information using a laptop or iPad during regular MS clinic visits: a pilot study. Multiple Sclerosis Journal 2011;17:S317.
Yon 2007 {published data only}
-
- Yon BA, Johnson RK, Harvey‐Berino J, Gold BC, Howard AB. Personal digital assistants are comparable to traditional diaries for dietary self‐monitoring during a weight loss program. Journal of Behavioral Medicine 2007;30(2):165‐75. - PubMed
Yu 2009 {published data only}
-
- Yu P, Courten M, Pan E, Galea G, Pryor J. The development and evaluation of a PDA‐based method for public health surveillance data collection in developing countries. International Journal of Medical Informatics 2009;78(8):532‐42. - PubMed
Zhang 2012 {published data only}
Zhu 2009 {published data only}
-
- Zhu ZH, Huang F, Wang WZ, Zhang JX, Ji Y, Zhang K. The psychometric properties of children's impact of event scale administered via mobile phone. Third International Conference on Bioinformatics and Biomedical Engineering. Beijing, China, 2009 Jun 11‐13:1‐3.
References to studies awaiting assessment
Anand 2015 {published data only (unpublished sought but not used)}
Benway 2013 {published data only}
-
- Benway B, McIntosh L, Black L, Morley J, Long S, Carter P, et al. Electronic data collection for patient‐reported outcomes in men with prostate cancer: assessing ease of use and patient satisfaction. Journal of Endourology 2013;27(Suppl 1):A62. [DOI: 10.1089/end.2013.2001] - DOI
Bjorner 2014a {published data only}
Bjorner 2014b {published data only}
-
- Bjorner JB, Rose M, Gandek B, Stone AA, Junghaenel DU, Ware JE Jr. Difference in method of administration did not significantly impact item response: an IRT‐based analysis from the Patient‐Reported Outcomes Measurement Information System (PROMIS) initiative. Quality of Life Research 2014;23(1):217‐27. [DOI: 10.1007/s11136-013-0451-4] - DOI - PMC - PubMed
Burke 2012 {published data only}
-
- Burke LE, Styn MA, Conroy MB, Ye L, Glanz K, Sevick MA, et al. Adherence to weight loss treatment across three self‐monitoring approaches and the association with weight change. Circulation 2012;125:A026.
Cunha‐Miranda 2014 {published data only}
-
- Cunha‐Miranda L, Santos H, Miguel C, Barcelos F, Silva C, Fernandes S, et al. Validation of touch‐screen questionnaires in spondyloarthropathies. Clinical and Experimental Rheumatology 2014;32:793‐4.
Nandkeshore 2013 {published data only}
-
- Nandkeshore H, Recker S, Patel P, Salapatek AM. Electronic patient acquisition tabletdemonstrates a high level of useracceptability and accommodation bypatients with allergic rhinitis studied inin environmental exposure chamber. Allergy: European Journal of Allergy and Clinical Immunology 2013;68(Suppl 97):360.
O'Gorman 2014 {published data only (unpublished sought but not used)}
-
- O'Gorman H, Mulhern B, Brazier J, Rotherham N. Comparing the equivalence of Eq‐5d‐5l across different modes of administration. Value in Health 2014;17:A517. - PubMed
Pfizer 2009 {unpublished data only}
-
- Pfizer. A Study to Compare Two Ways of Completing Pain and Sleep Questions and to Evaluate a New Daily Questionnaire for Assessing Fatigue in Fibromyalgia Patients. ClinicalTrials.gov 2009. [NCT00819624]
Schaffeler 2014 {published data only (unpublished sought but not used)}
-
- Schäffeler N, Wickert M, Wallwiener D, Zipfel S, Teufel M. Electronic psychooncological screening of cancer patients (ePOS): diagnostics and clinical pathways. Oncology Research and Treatment 2014;37(Suppl 1):106‐7.
References to ongoing studies
Khair 2015 {published and unpublished data}
-
- Khair K, Bladen M, Holland M. Physical function and quality of life in adolescents with haemophilia (SO‐FIT study). The Journal of Haemophilia Practice 2014;1(2):11‐4. [DOI: 10.17225/jhp.00018] - DOI
-
- Khair K, Holland M. Acceptability of patient related outcome measures by young people: the SO‐FIT study. Haemophilia 2015;21(Suppl 2):53‐4.
Kingston 2014 {published data only}
-
- Kingston D, McDonald S, Biringer A, Austin MP, Hegadoren K, McDonald S, et al. Comparing the feasibility, acceptability, clinical‐, and cost‐effectiveness of mental health e‐screening to paper‐based screening on the detection of depression, anxiety, and psychosocial risk in pregnant women: a study protocol of a randomized, parallel‐group, superiority trial. Trials 2014;15:3. [DOI: 10.1186/1745-6215-15-3] - DOI - PMC - PubMed
Additional references
Aanensen 2009
Adams 2014
-
- Adams P, Baumer EPS, Gay G. Staccato social support in mobile health applications. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems. 2014 Apr 26‐May 01:653‐62. [DOI: 10.1145/2556288.2557297] - DOI
Armstrong 2009
Barry 1992
-
- Barry MJ, Fowler FJ, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the Americal Urological Association. Journal of Urology 1992;148(5):1549‐57. - PubMed
Blundell 2010
Bowling 2005
-
- Bowling A. Mode of questionnaire administration can have serious effects on data quality. Journal of Public Health 2005;27(3):281‐91. - PubMed
Bowling 2009
-
- Bowling A. Research Methods in Health. 3rd Edition. New York, NY: Open University Press, 2009.
Boynton 2004
Bruce 2003
Bulloch 2009
Calin 1994
-
- Calin A, Garrett S, Whitelock H, Kennedy LG, O'Hea J, Mallorie P, et al. A new approach to defining functional ability in ankylosing spondylitys: the development of the Bath Ankylosing Spondylitis Functional Index. Journal of Rheumatology 1994;21(12):2281‐5. - PubMed
Carter 2000
-
- Carter Y, Shaw S, Thomas C. The Use and Design of Questionnaires. London: Royal College of General Practitioners, 2000.
Coons 2009
-
- Coons SJ, Gwaltney CJ, Hays RD, Lundy JJ, Sloan JA, Revicki DA, et al. ISPOR ePRO Task Force. Recommendations on evidence needed to support measurement equivalence between electronic and paper‐based patient‐reported outcome (PRO) measures: ISPOR ePRO Good Research Practices Task Force report. Value in Health 2009;12(4):419‐29. [DOI: 10.1111/j.1524-4733.2008.00470.x] - DOI - PubMed
Eaton 2004
-
- Eaton WW, Muntaner C, Smith C, Tien A, Ybarra M. Center for epidemiologic studies depression scale: review and revision (CESD and CESD‐R). In: Maruish ME editor(s). The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. 3rd Edition. London: Lawrence Erlbaum, 2004.
EndNote X5 [Computer program]
-
- Thomson Reuters. EndNote X5. Philadelphia, PA: Thomson Reuters, 2011.
EORTC QLQ‐C30
-
- EORTC Quality of Life. EORTC QLQ ‐ C30. http://groups.eortc.be/qol/eortc‐qlq‐c30 (accessed 31 October 2014).
Eyler 2013
Fan 2010
-
- Fan W, Yan Z. Factors affecting response rates of the web survey: a systematic review. Computers in Human Behaviour 2010;26(2):132‐9.
Gaggioli 2013
-
- Gaggioli A, Pioggia G, Tartarisco G, Baldus G, Corda D, Cipresso P, et al. A mobile data collection platform for mental health research. Personal and Ubiquitous Computing 2013;17(2):241‐51. [DOI: 10.1007/s00779-011-0465-2] - DOI
Garrett 1994
-
- Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. Journal of Rheumatology 1994;21(12):2286‐91. - PubMed
Ghersi 2009
Griffin 2012
Groves 2009
-
- Groves RM, Fowler FJ, Couper MP, Lepkowski JM, Singer E, Tourangeau R. Survey Methodology. 2nd Edition. Chichester: John Wiley & Sons, 2009.
Gwaltney 2008
Hicks 2001
-
- Hicks CL, Baeyer CL, Spafford PA, Korlaar I, Goodenough B. The Faces Pain Scale‐Revised: toward a common metric in pediatric pain measurement. Pain 2001;93(2):173‐83. - PubMed
Higgins 2001
-
- Higgins ET, Friedman RS, Harlow RE, Idson LC, Ayduk ON, Taylor A. Achievement orientations from subjective histories of success: promotion pride versus prevention pride. European Journal of Social Psychology 2001;31(1):3‐23. [DOI: 10.1002/ejsp.27] - DOI
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hosking 1995
-
- Hosking JD, Newhouse MM, Bagniewska A, Hawkins BS. Data collection and transcription. Controlled Clinical Trials 1995;16(2 Suppl):66S‐103S. - PubMed
Huckvale 2012
Ishii 2004
-
- Ishii K. Internet use via mobile phone in Japan. Telecommunications Policy 2004;28(1):43‐58.
Klasnja 2012
Lampe 1998
-
- Lampe AJ, Weiler JM. Data capture from the sponsoners' and investigators' perspectives: balancing quality, speed, and cost. Therapeutic Innovation & Regulatory Science 1998;32(4):871‐86. [DOI: 10.1177/009286159803200403] - DOI
Lane 2006
Lavrakas 2008
-
- Lavrakas PJ. Encyclopedia of Survey Research Methods. Los Angeles; London: SAGE Publications, Inc, 2008. [ISBN: 9781412918084]
Link 2014
-
- Link MW, Murphy J, Schober MF, Buskirk TD, Cilds JH, Tesfaye CL. Mobile Technologies for Conducting, Augmenting and Potentially Replacing Surveys: Report of the AAPOR Task Force on Emerging Technologies in Public Opinion Research. 25th April 2014. https://www.aapor.org/AAPORKentico/AAPOR_Main/media/MainSiteFiles/REVISE... (accessed 20 April 2015).
Manfreda 2008
-
- Manfreda L, Bosnjak M, Berzelak J, Haas I, Vehovar V. Web surveys versus other survey modes ‐ a meta‐analysis comparing response rates. International Journal of Market Research 2008;50(1):79‐104.
Marcano Belisario 2013
Mavletova 2014
-
- Mavletova A, Couper MP. Mobile web survey design: scrolling versus paging, SMS versus e‐mail invitations. Journal of Survey Statistics and Methodology 2014;2(4):498‐518. [DOI: 10.1093/jssam/smu015] - DOI
Oulasvirta 2012
-
- Oulasvirta A, Rattenbury T, Ma L, Raita E. Habits make smartphone use more pervasive. Personal and Ubiquitous Computing 2012;16:105‐14.
Radloff 1977
-
- Radloff LS. The CES‐D scale: a self‐report depression scale for research in the general population. Applied Psychological Measurement 1977;1:385‐401.
REDCap 2009
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodgers 2009
-
- Rodgers M, Sowden A, Petticrew M, Arai L, Roberts H, Britten N, et al. Testing methodological guidance on the conduct of narrative synthesis in systematic reviews: effectiveness of interventions to promote smoke alarm ownership and function. Evaluation 2009;15:49. [DOI: 10.1177/1356389008097871] - DOI
Rodrigues 2012
Sesto 2012
-
- Sesto ME, Irwin CB, Chen KB, Chourasia AO, Wiegmann DA. Effect of touch screen button size and spacing on touch characteristics of users with and without disabilities. Human Factors 2012;54(3):425‐36. - PubMed
Shih 2009
-
- Shih TH, Fan X. Comparing response rates in e‐mail and paper surveys: a meta‐analysis. Educational Research Review 2009;4(1):26‐40. [DOI: 10.1016/j.edurev.2008.01.003] - DOI
Tourangeau 2000
-
- Tourangeau R, Rips LJ, Rasinski K. The Psychology of Survey Response. Cambridge: Cambridge University Press, 2000.
Wallace 2014
Wells 2014
-
- Wells T, Bailey JT, Link MW. Comparison of smartphone and online computer survey administration. Social Science Computer Review 2014;32(2):238‐55. [DOI: 10.1177/0894439313505829] - DOI
References to other published versions of this review
Marcano Belisaro 2014
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

